Anthracyclines are commonly used antineoplastic drugs. However, their clinical utility is tempered by a dose-dependent risk of cardiotoxicity and congestive heart failure. Current preventive measures focus on dose reduction, use of less cardiotoxic anthracycline analogues and prophylactic use of dexrazoxane. Recent research has focused on early monitoring and risk stratification to identify patients that are ‘at risk’ for cardiotoxicity, using biochemical markers and the prophylactic use of novel cardioprotectants. This article reviews the clinical course, pathogenesis, cardiac monitoring and new concepts in diagnosing and preventing anthracycline cardiotoxicity.
Introduction
Anthracyclines have been used as efficacious antineoplastic agents for many haemopoietic and solid cancers since they were first isolated from the pigment-producing Streptomyces peuctius early in the 1960s.1 However, dose-dependent risk of cardiomyopathy and congestive heart failure has restricted their clinical utility.2 Risk factors for cardiotoxicity have been well investigated.3-5 They include cumulative dose greater than 550 mg/m2,3 age greater than 70,3 dose scheduling, mediastinal radiotherapy,6-9 previous cardiac disease,3,6 hypertension,3,8 whole-body hyperthermia,10 female sex11,12and combination therapy with other known cardiotoxic chemotherapeutic agents (i.e. trastuzumab and cyclophosphamide). The mechanism of cardiotoxicity appears to be multifactorial, however, the production of free radicals appears to be an important cause of myocyte damage and apoptosis.
Cardiotoxicity from anthracyclines can occur at any point during and subsequent to treatment. ‘Acute cardiotoxicity’ can occur immediately to weeks following treatment and usually presents as arrhythmias,13 ST and T wave abnormalities, pericarditis-myocarditis syndrome,14 and acute heart failure. More often patients develop ‘chronic cardiotoxicity,’ which presents as left ventricular dysfunction, chronic heart failure, and QT dispersion in the first year following treatment. ‘Late-onset cardiotoxicity’ develops following a prolonged asymptomatic period with heart failure presenting one year to decades following chemotherapy treatment. The incidence of late-onset ventricular dysfunction appears to increase in conjunction with the length of follow-up (figure 1).15
Given the potential for late cardiotoxicity, cardiac monitoring of patients treated with anthracyclines needs to be a lifelong process. The standard clinical approach includes cardiac assessment before therapy begins, monitoring during treatment and follow-up after treatment completion.5
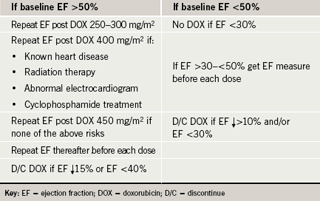
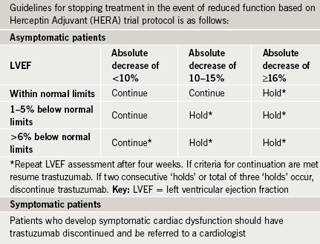
The American Cardiology Committee of the Children’s Cancer Study has published a summary of paediatric monitoring guidelines using electrocardiogram (ECG), echocardiography and radionuclide angiography to evaluate for cardiotoxicity.16 In adults, the assessment of cardiac function by serial echocardiography and/or radionuclide angiography (RNA) is a well-established cost-effective method for preventing morbidity and mortality related to anthracycline chemotherapy.17 Schwartz et al. have developed a monitoring algorithm (table 1) with scheduled frequent ejection fraction measurements, that, when used, has demonstrated a four-fold reduction in the risk of congestive heart failure.18 The United Kingdom National Cancer Research Institute has developed separate guidelines for cardiac monitoring and the use of trastuzumab, another cardiotoxic chemotherapeutic agent (table 2).19 Recent research focusing on the use of strain echography, an echo technique that looks at the deformation of the heart muscle as it contracts, has been promising as a more sensitive modality for detecting early cardiotoxicy,20 but further research is needed.
New developments in risk stratification and early monitoring for cardiotoxicity: the use of biochemical markers
Despite routine tracking of ejection fractions to adjust drug dosing, some patients still develop severe left ventricular dysfunction. Uncertainty of the risk of heart failure has led many oncologists to routinely avoid high-dose anthracyclines. While lower dosing reduces the incidence of cardiac dysfunction, this approach deprives many patients of the full benefit of these chemotherapeutic agents. In reality not all cancer patients develop cardiac events (Cardinale et al.21 reported 16% total cumulative cardiac events). This marked individual cardiac response to doxorubicin may be due to genetic factors and/or differing susceptibility to drug-induced oxidative stress. Better biomarkers to detect high- versus low-risk patients are needed to enable physicians to individualise chemotherapy regimens.
Troponin
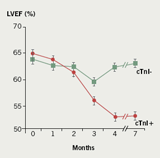
Recently, troponin has been used as a marker of myocardial injury to risk stratify patients undergoing anthracycline treatment. Initial animal studies found troponin T to be an early and sensitive marker of anthracycline-induced cardiotoxicity.22 Cardinale et al. pioneered the use of troponins in the prediction of anthracycline cardiotoxicity in adults in two clinical studies. In the first study, troponin I levels were measured in 204 patients before, immediately after, and 12, 24, 36 and 72 hours after each cycle of high-dose chemotherapy. Patients were classified as either troponin I ‘positive’ or ‘negative’ and then had comparison of echocardiographic derived left ventricular ejection fraction (LVEF) between groups. Both groups demonstrated significant LVEF impairment at three months. However, the troponin I ‘negative’ group LVEF returned to baseline by four to seven months, whereas, the troponin I ‘positive’ group had a persistently reduced LVEF at seven months (figure 2).
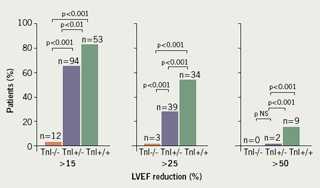
A second study of 703 patients measured troponin before, soon after (immediately, 12, 24, 36 and 72 hours), and late after (one month after dosing and just prior to the next scheduled drug treatment) each course of chemotherapy. Patients had one of three patterns of troponin response: Group 1. troponin ‘negative’ both early (1–72 hours) and late (four weeks); Group 2. troponin ‘positive’ early, but troponin ‘negative’ late; Group 3. troponin ‘positive’ both early and late. Group 3 went on to have the highest rate of cardiac events and the greatest reduction in LVEF over three years (figure 3).21
These studies show that troponin I leak patterns following chemotherapy can risk stratify patients before structural changes in cardiac function are evident. Though frequent sampling of troponin after each drug infusion may be impractical, elevated troponin at one-month post each dose appears to identify at-risk patients. The Cardinale data, however, are specific to patients receiving high-dose doxorubicin on an every-four-week protocol. Since many oncology units administer chemotherapy at one to two-week intervals, additional studies are needed to clarify the use of troponin as a biomarker of potential cardiotoxicity.
Alternatively, identification of genomic markers or measures of pro-oxidant stress response to chemotherapy may better risk stratify cancer chemotherapy patients.
Prevention
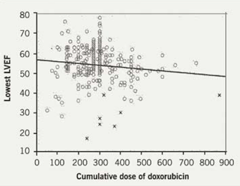
While dose adjustment is the primary approach to prevent cardiac dysfunction, a significant number of patients still develop severe cardiotoxicity at doses well below 550 mg/m2 as shown in figure 4.17Historically, the use of anthracycline analogues and the use of dexrazoxane have been used to protect patients who show evidence of early toxicity at low-to-medium doxorubicin doses.
Anthracycline analogues
Over the last several decades, researchers have attempted to develop analogues that are superior in terms of activity and cardiotoxicity. Epirubicin, idarubicin, and mitoxantrone have been shown in clinical trials to lower the rate of cardiotoxicity.24 A comparative study examining the tumour effect and cardiotoxicity of doxorubicin and epirubicin showed that epirubicin is equally active and less cardiotoxic than doxorubicin.25
Liposomal analogues
Liposomal analogues of anthracyclines have been developed to reduce drug toxicity while preserving antineoplastic effects by selectively perfusing into tumour tissue. Three liposomal anthracyclines are currently being investigated: liposomal daunorubicin, liposomal doxorubicin and pegylated liposomal doxorubicin.26 Early research has indicated that the risk of developing cardiotoxicity is significantly higher for patients receiving doxorubicin than those receiving pegylated liposomal doxorubicin.27
New approaches to liposomal analogues
Investigators are currently evaluating the use of the bacterium Clostridium Novyi-NT in selectively delivering liposomal doxorubicin to tumours. This organism is an obligate anaerobe that can colonise the hypoxic regions of tumours and cause partial destruction of a cancer by its membrane disrupting properties while enhancing the release of liposomal doxorubicin directly into tumours. Using mouse tumour models, injection of Clostridium Novyi-NT spores prior to administration of liposomal doxorubicin-enhanced tumour response and resulted in higher drug levels within the tumour.28,29 This strategy opens new possibilities in the use and development of novel liposomal analogues and their delivery mechanisms.
Cardioprotectants
An adjunctive approach to preventing anthracycline toxicity is blunting drug-induced pro-oxidant stress. Dexrazoxane is the only United States Food and Drug Administration approved agent for reducing the incidence and severity of cardiomyopathy associated with doxorubicin administration in women with metastatic breast cancer. It is recommended for use in patients who received greater than 300 mg/m2 of doxorubicin or greater than 550 mg/m2 of epirubicin and who require further administration of these agents for advanced, but anthracycline-sensitive, cancers.30
New prevention strategies
In addition to new biomarkers for risk stratification, there are new potential approaches to prevention of anthracycline cardiotoxicity. These include the use of angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs) and carvedilol.
ACE inhibitors
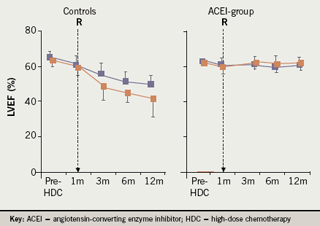
ACE inhibitors may prevent doxorubicin cardiotoxicity by reducing left ventricular remodelling and limiting oxidative stress. Cardinale et al. assessed the benefits on left ventricular dysfunction in high-risk patients (troponin positive) treated with enalapril. One hundred and fourteen patients with elevated troponin I soon after high-dose chemotherapy were randomised to enalapril 20 mg/day versus placebo. Treatment was started one month following chemotherapy and continued for one year. Only the placebo patients developed reduced left ventricular function (figure 5), and adverse cardiac events including congestive heart failure, arrhythmias needing treatment, and two cardiac deaths.31
The future of prevention with ACE inhibitors looks extremely promising. However, further multi-centre studies are needed to confirm the benefits of ACE inhibitors and determine the optimal timing for initiation of ACE inhibitors.
Angiotensin receptor blockers
Angiotensin, a peptide of the renin–angiotensin system, plays a significant role in doxorubicin-induced heart disease. ARBs have been found to have intrinsic antioxidant effects and these may mediate a cardioprotective effect against anthracycline toxicity. Research has shown that doxorubicin does not cause cardiotoxicity in angiotensin II type I receptor gene knock-out mice and administration of ARBs to mice can prevent daunorubicin-induced cardiomyopathy.32 Nakamae and colleagues investigated whether valsartan can inhibit acute cardiotoxicity in 40 patients with untreated non-Hodgkin lymphoma following treatment with doxorubicin. They found that valsartan significantly reduced changes in the left ventricular end-diastolic diameter, the QTc interval and QTc dispersion on the ECG.33
Carvedilol
Carvedilol blocks beta1, beta2 and alpha1 adrenoceptors and has potent antioxidant and anti-apoptetic properties. Early research in animals has shown that the use of carvedilol can prevent chemotherapeutic cardiotoxicity.34 Kalay and associates conducted the first human clinical trial investigating the prophylactic use of carvedilol in this clinical setting. Fifty patients receiving anthracyclines were randomised to either the carvedilol group or control group. The carvedilol group was started on 12.5 mg carvedilol once daily prior to chemotherapy and continued for six months. At the end of six months the mean ejection fraction of the carvedilol group was similar to the baseline ejection fraction (70.5 vs. 69.7; p=0.3), however, the control group ejection fraction was significantly lower (68.9 vs.52.4; p<0.001).35 Further large randomised trials are needed to verify the benefit of carvedilol in reducing cardiotoxicity in chemotherapy patients.
Conclusions
Anthracycline cardiotoxicity continues to be a serious problem. Patients undergoing anthracycline treatment need regular monitoring of LVEF prior to, during and after treatment. Currently there is no universal monitoring guideline. The risk factors for anthracycline cardiotoxicity have been well known for a long time but are not sufficient to prevent many cases of cardiomyopathy. Only recently has it been shown that biochemical markers, such as troponin T and troponin I, may be used for early detection of those at risk. Other future avenues of prevention include identifying genetic markers of increased susceptibility to anthracycline cardiotoxicity. Once the specific patients at increased risk are identified, they may be protected with agents such as ACE inhibitors, ARBs and carvedilol, such that they may continue receiving maximal doses of life-saving chemotherapies with minimal cardiotoxic effects.
Conflict of interest
None declared.
Key messages
- Anthracyclines are commonly used in the treatment of cancer, however, they are associated with a dose-dependent risk of cardiotoxicity and congestive heart failure
- Prevention of cardiotoxicity is important to ensure that cancer patients receive the maximal benefits of chemotherapy while minimising risk
- Current measures include reducing anthracycline dosages, using less cardiotoxic anthracycline analogues and prophylactic use of dexrazoxane
- Biochemical markers to identify patients ‘at risk’ have been identified
- ACE inhibitors, angiotensin receptor blockers and carvedilol may have a role in preventing cardiotoxicity
References
- Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev2004;56:185–229.
- Lefrak EA, Pitha J, Rosenheim S, Gottlieb JA. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer 1973;32:302–14.
- Von Hoff DD, Layard MW, Basa P et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med 1979;91:710–17.
- Levitt G. Cardioprotection. Br J Haematol 1999;106:860–9.
- Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med 1998;339:900–05.
- Praga C, Beretta G, Vigo PL et al. Adriamycin cardiotoxicity: a survey of 1273 patients. Cancer Treat Rep 1979;63:827–34.
- Billingham ME, Bristow MR, Glatstein E, Mason JW, Masek MA, Daniels JR. Adriamycin cardiotoxicity: endomyocardial biopsy evidence of enhancement by irradiation. Am J Surg Pathol1977;1:17–23.
- Minow RA, Benjamin RS, Lee ET, Gottlieb JA. Adriamycin cardiomyopathy—risk factors. Cancer 1977;39:1397–402.
- Pihkala J, Saarinen UM, Lundstrom U et al. Myocardial function in children and adolescents after therapy with anthracyclines and chest irradiation. Eur J Cancer 1996;32A:97–103.
- Kim YD, Lees DE, Lake CR et al. Hyperthermia potentiates doxorubicin-related cardiotoxic effects. JAMA 1979;241:1816–17.
- Lipshultz SE, Lipsitz SR, Mone SM et al. Female sex and drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med 1995;332:1738–43.
- Silber JH, Jakacki RI, Larsen RL, Goldwein JW, Barber G. Increased risk of cardiac dysfunction after anthracyclines in girls. Med Pediatr Oncol 1993;21:477–9.
- Steinberg JS, Cohen AJ, Wasserman AG, Cohen P, Ross AM. Acute arrhythmogenicity of doxorubicin administration. Cancer 1987;60:1213–18.
- Harrison DT, Sanders LA. Letter: Pericarditis in a case of early daunorubicin cardiomyopathy. Ann Intern Med 1976;85:339–41.
- Steinherz LJ, Steinherz PG, Tan CT, Heller G, Murphy ML. Cardiac toxicity 4 to 20 years after completing anthracycline therapy. JAMA 1991;266:1672–7.
- Steinherz LJ, Graham T, Hurwitz R et al. Guidelines for cardiac monitoring of children during and after anthracycline therapy: report of the Cardiology Committee of the Childrens Cancer Study Group. Pediatrics 1992;89:942–9.
- Mitani I, Jain D, Joska TM, Burtness B, Zaret BL. Doxorubicin cardiotoxicity: prevention of congestive heart failure with serial cardiac function monitoring with equilibrium radionuclide angiocardiography in the current era. J Nucl Cardiol 2003;10:132–9.
- Schwartz RG, McKenzie WB, Alexander J et al. Congestive heart failure and left ventricular dysfunction complicating doxorubicin therapy. Seven-year experience using serial radionuclide angiocardiography. Am J Med 1987;82:1109–18.
- UK Clinical Guidelines for the Use of Adjuvant Trastuzumab (Herceptin) With or Following Chemotherapy in HER2-positive Early Breast Cancer. NCRI Breast Clinical Studies Group Clinical Studies Group. 14th December 2005. Available from: http://www.dh.gov.uk/en/Healthcare/NationalServiceFrameworks/Cancer/DH_4126383
- Ganame J, Claus P, Uyttebroeck A et al. Myocardial dysfunction late after low-dose anthracycline treatment in asymptomatic pediatric patients. J Am Soc Echocardiogr 2007;20:1351–8.
- Cardinale D, Sandri MT, Colombo A et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation 2004;109:2749–54.
- Koh E, Nakamura T, Takahashi H. Troponin-T and brain natriuretic peptide as predictors for adriamycin-induced cardiomyopathy in rats. Circ J 2004;68:163–7.
- Cardinale D, Sandri MT, Martinoni A et al. Left ventricular dysfunction predicted by early troponin I release after high-dose chemotherapy. J Am Coll Cardiol 2000;36:517–22.
- Weiss RB. The anthracyclines: will we ever find a better doxorubicin? Semin Oncol 1992;19:670–86.
- Hortobagyi GN, Yap HY, Kau SW et al. A comparative study of doxorubicin and epirubicin in patients with metastatic breast cancer. Am J Clin Oncol 1989;12:57–62.
- Wouters KA, Kremer LC, Miller TL, Herman EH, Lipshultz SE. Protecting against anthracycline-induced myocardial damage: a review of the most promising strategies. Br J Haematol2005;131:561–78.
- O’Brien ME, Wigler N, Inbar M et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol 2004;15:440–9.
- Juliano R. Bugging tumors to put drugs on target. N Engl J Med 2007;356:954–5.
- Cheong I, Huang X, Bettegowda C et al. A bacterial protein enhances the release and efficacy of liposomal cancer drugs. Science 2006;314:1308–11.
- Systemic Treatment Disease Site Group. Seymour L BV. Use of dexrazoxane as a cardioprotectant in patients receiving doxorubicin or epirubicin chemotherapy for the treatment of cancer [full report]. Toronto (ON): Cancer Care Ontario (CCO), 2004;23 p (Practice guideline report; no. 12-15).
- Cardinale D, Colombo A, Sandri MT et al. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation2006;114:2474–81.
- Soga M, Kamal FA, Watanabe K et al. Effects of angiotensin II receptor blocker (candesartan) in daunorubicin-induced cardiomyopathic rats. Int J Cardiol 2006;110:378–85.
- Nakamae H, Tsumura K, Terada Y et al. Notable effects of angiotensin II receptor blocker, valsartan, on acute cardiotoxic changes after standard chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisolone. Cancer 2005;104:2492–8.
- Spallarossa P, Garibaldi S, Altieri P et al. Carvedilol prevents doxorubicin-induced free radical release and apoptosis in cardiomyocytes in vitro. J Mol Cell Cardiol 2004;37:837–46.
- Kalay N, Basar E, Ozdogru I et al. Protective effects of carvedilol against anthracycline-induced cardiomyopathy. J Am Coll Cardiol 2006;48:2258–62.
