This year’s European Society of Cardiology (ESC) congress, held in Barcelona, Spain, from August 29th–September 2nd 2009, was awash with promising new data, including results from two mega studies – RE-LY and PLATO – which were described as ‘ground breaking’.
RE-LY: a warfarin replacement finally delivers the goods
A new oral drug that does not need monitoring showed benefits over warfarin in patients with atrial fibrillation in the phase 3 RE-LY (Randomised Evaluation of Long-Term Anticoagulation Therapy) trial, suggesting that a viable replacement for warfarin has finally been found.
In the trial, dabigatran (Pradaxa) given at a dose of 110 mg was associated with similar rates of stroke and systemic embolism to warfarin but with less major bleeding. A higher dose of dabigatran (150 mg) produced lower rates of stroke and systemic embolism than warfarin with similar rates of major bleeding.
Dabigatran, a thrombin inhibitor, is one of several new oral anticoagulants being developed. It has recently been made available in Europe for the prevention of venous thromboembolism during hip- and knee-replacement surgery.
Presenting the RE-LY data at the Barcelona meeting, Professor Stuart Connolly (McMaster University, Canada) said: “Although researchers have been looking for a replacement for warfarin for several decades, nothing has been successful. This is the first time in more than 50 years that a new oral anticoagulant has been developed which has been found to be both safer and more effective than existing therapy”.
The RE-LY results were greeted with much excitement at the ESC meeting, with the terms “breakthrough” and “groundbreaking” being used. The prospect of a replacement for warfarin that does not need monitoring could make life significantly easier for the millions of patients taking warfarin for atrial fibrillation and enable many more, who cannot cope with the demands of monitoring, able to take an effective drug for stroke prevention.
Designated discussant of the RE-LY trial at the ESC meeting, Dr John Camm (St George’s University of London) summed up the enthusiasm, saying: “Dabigatran seems not to be merely a superior therapy, but we must probably regard this drug as a stimulus to a paradigm change in the antithrombotic management of atrial fibrillation”.
But some caution was voiced over the need for longer-term safety follow-up, given the bad experience seen with the last warfarin replacement candidate, ximelegatran, which had to be removed from the market shortly after launch because of liver toxicity. No signs of liver toxicity were seen in the RE-LY trial, the main side-effect of dabigatran being dyspepsia.
The RE-LY study is the largest outcomes study in atrial fibrillation ever conducted. It was carried out in 44 countries and randomised 18,113 patients with atrial fibrillation and at least one other risk factor for stroke to receive dabigatran at either 110 mg or 150 mg twice daily or warfarin adjusted to an INR of 2.0 to 3.0. Aspirin was taken throughout the trial by about 20% of patients. The median duration of the follow-up period was two years. The primary end point was stroke or systemic embolism.
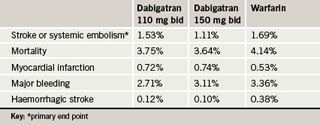
Results (table 1) showed that the higher dose of dabigatran significantly reduced the risk of stroke by 34% compared to warfarin (p<0.001), with a similar bleeding risk. The lower dose had a similar effect to warfarin in the prevention of stroke, but with significantly less major bleeding. The risk of myocardial infarction was increased with dabigatran, which just reached significance in the high-dose group. Both dabigatran groups showed significantly lower rates of haemorrhagic strokes compared with warfarin.
The only adverse effect that was significantly more common with dabigatran than with warfarin was dyspepsia, which occurred in 5.8% of the warfarin group, 11.8% of low-dose dabigatran patients and 11.3% of the high-dose dabigatran patients.
The RE-LY results were published online in the New England Journal of Medicine (10.1056/NEJMoa0905561) to correspond with their release in Barcelona.
In an accompanyng editorial, Dr Brian Gage (Washington University, St Louis, US) suggests that patients already taking warfarin with excellent INR control should not switch treatments. But many other patients who have atrial fibrillation and at least one additional risk factor for stroke could benefit from dabigatran.
Dr Gage notes that increases in liver enzymes were similar in the two dabigatran groups and the warfarin group, and much lower than that seen with ximelagatran, adding that longer-term hepatic risks of dabigatran are being quantified in a follow-up study.
He says that patients who had a creatinine clearance of less than 30 ml per minute or liver disease were excluded from the RE-LY study and should not receive the drug. Non-compliant patients also were excluded, and they might receive less benefit from dabigatran, because the longer half-life of warfarin could provide them with a more consistent anticoagulant effect, he pointed out.
Other thrombin inhibitor studies in AF
Daiichi-Sankyo announced at the meeting that it was beginning a phase 3 study – ENGAGE AF-TIMI 48 – with the Xa inhibitor edoxaban for stroke prevention in atrial fibrillation. This double-blind study will be carried out in 16,500 patients with a 24-month follow-up comparing two different doses of edoxaban (30 mg and 60 mg qd) versus warfarin in individuals with atrial fibrillation at moderate to high risk of stroke. Phase 2 studies with the agent have shown a dose-dependent anticoagulation effect, and that 30 mg and 60 mg doses once daily were well tolerated, with bleeding being similar or lower than warfarin. Twice-daily dosing with edoxaban was associated with increased bleeding versus warfarin.
Other studies ongoing in this area include the phase 3 ROCKET AF study looking at rivaroxaban (20 mg once daily) versus warfarin in the prevention of stroke and non-CNS systemic embolism in patients with non-valvular atrial fibrillation. During the meeting, Bayer announced that it had completed patient enrolment into the study, involving 14,269 patients in 45 countries. A further study, ARISTOTLE, funded by Bristol Myers Squibb is comparing apixaban (5 mg twice daily) to warfarin also in the prevention of stroke in 15,000 patients with non-valvular atrial fibrillation. Both studies are expected to complete next year.
PLATO: ticagrelor beats clopidogrel in ACS
Astra Zeneca’s new antiplatelet agent, ticagrelor (Brilinta®), reduced the rate of cardiovascular events compared to clopidogrel without an increase in major bleeding in the large-scale PLATO (Platelet Inhibition and Patient Outcomes) trial in acute coronary syndrome (ACS) patients.
“Ticagrelor is the first antiplatelet therapy to achieve a significant reduction in cardiovascular mortality in ACS patients versus clopidogrel and perhaps, most importantly, without an increase in major bleeding,” commented chief investigator Professor Lars Wallentin (Uppsala Clinical Research Center, Sweden). “PLATO has redefined what is possible in the prevention of recurrent events in patients with acute coronary syndromes.”
Ticagrelor is the first direct acting and reversibly binding oral ADP receptor antagonist, which, like clopidogrel and prasugrel, targets the P2Y12 receptor. But ticagrelor is from a different chemical class than clopidogrel and prasugrel and has the advantage of producing rapid and reversible inhibition of platelet aggregation, which is seen as an advantage in the acute situation.
In the PLATO study, 18,624 ACS patients were randomised to ticagrelor (180 mg loading dose then a 90 mg twice-daily maintenance dose) or clopidogrel (300–600 mg loading then a 75 mg twice-daily maintenance dose). All patients received concomitant aspirin.
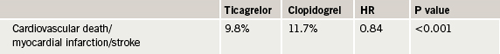
The primary end point (a composite of death from vascular causes, myocardial infarction, or stroke at 12 months) was significantly reduced in the ticagrelor group (table 1). Ticagrelor demonstrated a consistent benefit across multiple secondary efficacy end points including cardiovascular death and total mortality; myocardial infarction; the composite of myocardial infarction, stroke, and total mortality; and a composite of cardiovascular death, myocardial infarction, stroke, transient ischaemic attack, recurrent cardiac ischaemia, severe recurrent cardiac ischaemia, and other arterial thrombotic events, Professor Wallentin reported.
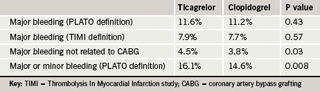
These benefits were obtained without an increase in major bleeding as defined in the PLATO study. Ticagrelor was, however, associated with a higher rate of major bleeding not related to coronary artery bypass grafting (CABG) than clopidogrel. In addition, there was a small increase in major and minor bleeding combined (table 2).
In terms of other side-effects, ticagrelor was associated with more frequent pauses in heart rhythm on continuous ECG monitoring while in hospital, but these were not associated with any symptoms or clinical consequences. Transient symptoms of dyspnoea were also reported more often by patients on ticagrelor but only one in 100 ticagrelor-treated patients overall stopped taking study medication due to dyspnoea.
Professor Wallentin noted that for every 1,000 patients, use of ticagrelor instead of clopidogrel, for up to one year, would lead to 14 fewer deaths, or 11 fewer myocardial infarctions, or eight fewer cases of stent thrombosis, without an increase in major bleeds.
The PLATO results were well received at Barcelona, with most experts saying that the drug did seem to have benefits over both clopidogrel and prasugrel, with its “quick on-off action” particularly attractive in the acute situation. This means that if a patient has to go to emergency by-pass surgery, the antiplatelet effect can be stopped rapidly which should translate into a lower bleeding risk.
In his remarks about the study, designated discussant of PLATO at the ESC Hotline, Dr Steen Kristensen (Aarhus University Hospital, Denmark), commented that ticagrelor is “a promising drug, with better efficacy than clopidogrel”.
The PLATO study was published online on August 30th in the New England Journal of Medicine (10.1056/NEJMoa0904327) to coincide with its presentation at the ESC.
CURRENT-OASIS 7: doubling the clopidogrel dose around time of PCI reduces event rates
Doubling the doses of the antiplatelet agent, clopidogrel (Plavix®), for a week around the time of percutaneous coronary intervention (PCI) reduced complications in the CURRENT-OASIS 7 in patients with unstable angina/myocardial infarction (MI).
“The superiority of the high-dose clopidogrel regimen in reducing stent thrombosis and related heart attacks in those undergoing PCI is clear in our study and will be of great relevance to interventional cardiologists,” said Dr Shamir Mehta (McMaster University, Canada), who was principal investigator of the trial.
The trial also evaluated the optimal dose of aspirin and found that 300 mg of aspirin resulted in similar outcomes to 100 mg of aspirin and was not associated with higher rates of bleeding. There was also no benefit of the higher dose of clopidogrel in patients not undergoing PCI.
CURRENT-OASIS 7 (Clopidogrel Optimal Loading Dose Usage to Reduce Recurrent Events/Optimal Antiplatelet Strategy for Interventions) enrolled 25,087 patients scheduled to undergo angiography within 72 hours of arriving at hospital with unstable angina or an MI. Of these, about 17,000 underwent PCI. As soon as possible after their arrival, patients were randomised to high-dose clopidogrel (600 mg loading followed by 150 mg once a day for seven days, then 75 mg daily thereafter), or standard dose clopidogrel (300 mg on day one, then 75 mg once a day thereafter). Patients in both groups were randomly assigned to aspirin, either high-dose (300–325 mg once daily) or low-dose regimen (75–100 mg once daily).
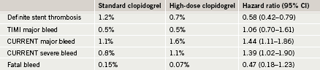
Results showed no significant difference in the overall cohort of patients between the high and standard doses of clopidogrel (table 1). But in the PCI subgroup there was a significant 15% reduction in cardiovascular death, MI and stroke. This reduction was driven by a 22% reduction in the risk of MI. In addition, there was a significant 42% reduction in the risk of definite stent thrombosis.
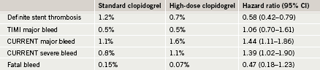
There was no difference between the two clopidogrel doses in TIMI (Thrombolysis in Myocardial Infarction) major bleeding, but there was a significant increase with the high dose when the CURRENT major and severe bleeding definition was used, which Dr Mehta said was more sensitive than TIMI (table 2). This was driven by an increased need for red blood cell transfusions. But there was no difference in fatal bleeding, intracranial hemorrhage, or CABG. Dr Mehta reported that for every 1,000 patients with ACS undergoing PCI, doubling the dose of clopidogrel will prevent an additional six MIs and seven stent thromboses with an excess of three severe bleeds.
“This is a simple thing to institute rapidly – just going from one pill to two pills per day, the cost implications are virtually negligible and the benefits are large. So this manoeuvre could improve patient outcomes in PCI immediately,” Dr Mehta commented.
Designated discussant of the study, Dr Frans Van de Werf (University of Leuven, Belgium) agreed that there was a favourable net clinical benefit with the higher dose clopidogrel in the PCI patients. “The price to pay in terms of bleeding complications is small and certainly acceptable,” he said.
Aspirin findings
In the aspirin part of the study, there was no significant difference in either efficacy or bleeding with high-dose versus low-dose aspirin, although there was a trend towards a reduction in the primary end point with the high dose (table 3).
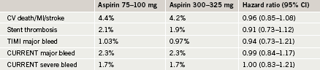
Dr Mehta noted that low-dose aspirin has been recommended mainly because of concerns about bleeding risk with higher doses, but these data suggest that “there is really no reason not to use high-dose aspirin, at least for 30 days after placing a stent”. Dr Van de Werf disagreed with Dr Mehta on the aspirin message, however, saying: “These data provide no support for the use of the higher dose”.
No to aspirin use in asymptomatic atherosclerosis
The routine use of aspirin for the primary prevention of vascular events in people with asymptomatic disease is not recommended, according to the results of the AAA (Aspirin for Asymptomatic Atherosclerosis) study. The study is the first placebo-controlled randomised trial designed to determine the effect of aspirin in asymptomatic atherosclerosis diagnosed by a low ankle brachial index (ABI) measurement, and found no benefit of aspirin over placebo. Aspirin did, however, increase bleeding.
Joint first author Professor Gerry Fowkes (Wolfson Unit for Prevention of Peripheral Vascular Diseases, Edinburgh) said: “It is possible that in the general population, aspirin could produce a smaller reduction in vascular events than this trial was designed to detect, but it is questionable whether such an effect, together with aspirin related morbidity, would justify the additional resources and health care requirements of an ABI screening programme.”
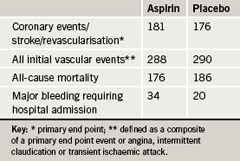
The investigators measured ABI in 28,980 men and women free of clinically evident cardiovascular disease to find 3,350 who had a low measurement (≤0.95). These patients were randomised to once daily aspirin 100 mg or placebo, and followed for a mean of 8.2 years. Outcomes were ascertained by annual contact, GP records, hospital discharge records, and death notification. There was no difference in the primary end point – a composite of coronary events/stroke/revascularisation – or in two secondary end points between the two groups (table 1).
“These results would suggest that using the ABI as a tool to screen individuals free of cardiovascular disease in the community is unlikely to be beneficial if aspirin is the intervention to be used in those found to be at higher risk. Other more potent antiplatelets might be considered, but only if increased effectiveness in avoiding ischaemic events is not matched by increased bleeding,” Dr Fowkes said.
He noted that 40% of patients in the AAA trial did not take their aspirin as prescribed over the duration of the trial, and such a low compliance rate could have affected the results. “Whether aspirin is beneficial in clinical practice among patients who have a low ankle-brachial index and who are fully compliant is unknown, and so our results cannot be extrapolated to that situation,” he said.
Designated discussant of the study, Dr Carlo Patrono (Catholic University School of Medicine, Rome, Italy) pointed out that the results of the AAA trial conflicted with those of a meta-analysis from the Antithrombotic Trialists’ (ATT) collaboration, published earlier this year, which showed a 12% relative risk reduction in serious cardiovascular events with aspirin in this population. He added that the authors of the two studies are collaborating to try and establish why they showed different results.
TRIANA : primary angioplasty ‘probably’ better than lysis in very elderly
Primary angioplasty appeared to be preferable to thrombolysis in the treatment of very old patients with ST-elevation myocardial infarction (STEMI) in the TRIANA (Primary Angioplasty versus Fibrinolysis in the Very Elderly) study.
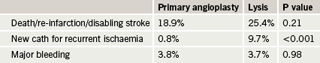
The trial was performed in 226 Spanish STEMI patients all aged 75 years or older and who were within six hours of symptom onset. It was stopped early because of slow patient recruitment – clinicians were reluctant to enrol patients largely because of concerns about bleeding with thrombolysis. It was therefore underpowered. Results (table 1) found no statistical difference between primary angioplasty and lysis (weight-adjusted tenecteplase) in the primary end point – the incidence of death, reinfarction or disabling stroke at 30 days. However, in a pre-specified secondary end point there was a significantly lower need of new catheterisation for recurrent cardiac ischaemia in the primary angioplasty arm.
Chief investigator, Professor Héctor Bueno (General Universitario “Gregorio Marañón”, Madrid, Spain) said there was no clear evidence that thrombolysis, which is considered controversial in older patients because of their increased bleeding risk, was unsafe in this population whose median age was 81 years. Results showed no intracranial bleeding directly related to the use of thrombolysis, and no significant differences between groups in major bleeding (table 1), or need for transfusions, and there was also was no increase in renal failure associated with primary angioplasty (6.1% versus 7.5%), a feared complication of catheterisation in older patients.
Professor Bueno added: “All efficacy outcomes showed concordant trends in favour of primary angioplasty, suggesting that the potential advantage of an invasive strategy over thrombolysis in very old patients is because of its greater efficacy rather than its superior safety”. At 12 months, the superiority of primary angioplasty with regard to recurrent ischaemia continued to be significant, he added.
Discussant of the study, Dr Dariusz Dudek (Institute of Cardiology, Krakow, Poland), said TRIANA confirms results of the SENIOR PAMI trial, conducted in the US, and is in line with the guidelines which have no age limits for primary PCI.
MADIT-CRT: Pacing improves outcomes in mild heart failure
Patients with ventricular dyssynchrony by ECG and only mild heart failure had a better outcome with a cardiac resynchronisation device with defibrillator (CRT-ICD), rather than a standard cardioverter defibrillator (ICD) alone in the MADIT-CRT (The Multicentre Automatic Defibrillator Implantation Trial – Cardiac Resynchronisation Therapy) study.
“This study provides evidence that preventive CRT-ICD therapy decreases the risk of heart-failure events in vulnerable patients with ischaemic or non-ischaemic heart disease who have minimal heart-failure symptoms but a wide QRS complex,” lead investigator, Dr Arthur Moss (University of Rochester Medical Center, New York, USA) said.
Cardiac resynchronisation therapy is already indicated for use in patients with severe heart failure, but the current study was investigating whether CRT would prevent the development of heart failure in less severe heart failure by intervening early in the course of the disease.
The trial was conducted in 1,820 patients for whom a primary-prevention implantable cardioverter defibrillator was already indicated. They had class I or II heart failure (no or mild symptoms) with an ejection fraction less than 30% and a QRS duration of more than 130 ms on ECG.
Patients were randomised to receive CRT-ICD or an ICD alone, with everyone receiving optimal medical therapy.
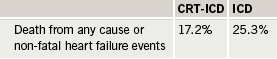
Results showed that over a follow-up averaging 2.4 years, there was a 34% reduction in the risk of all-cause mortality or heart failure events (the primary end point) in the CRT-ICD group (table 1).
A prespecified subgroup analysis showed that the CRT-ICD benefit was driven by a 52% risk reduction in patients with QRS duration of >150 ms versus shorter QRS. There was also a significant interaction by sex, with women showing greater benefit than men.
Dr Moss commented: “Cardiac resynchronisation therapy was dramatically effective in this large study population. These results validate a new indication for cardiac resynchronisation therapy in the prevention of heart failure in at-risk asymptomatic or mildly symptomatic cardiac patients. It seems likely that this preventive CRT-D therapy will have widespread application and utilisation.”
The MADIT-CRT findings were published online on 1st September in the New England Journal of Medicine (10.1056/NEJMoa0906431) to coincide with their release at the ESC meeting. In an accompanying editorial, Dr Mariell Jessup (University of Pennsylvania, US) suggests that: “Given the benefit primarily in patients with a QRS duration of >150 ms and cost-effectiveness concerns if CRT use is broadened based on MADIT-CRT, it appears prudent that any expanded indications to include patients with milder heart failure be limited to those with such very wide QRS complexes and a history of marked symptoms that were under control with medical therapy”.
Kyoto Heart Study: should ARBs be first-line in high-risk hypertensives?
Addition of the angiotensin II receptor antagonist (ARB), valsartan, to conventional antihypertensive treatment to improve blood pressure control was associated with an improved cardiovascular outcome in Japanese hypertensive patients at high risk of cardiovascular events in the Kyoto Heart Study.
In the study, more than 3,031 Japanese patients with uncontrolled hypertension and one or more cardiovascular risk factors were randomised to receive either additional treatment with valsartan or non-ARB conventional therapies.
The primary end point was a composite of defined cardio- or cerebrovascular events including stroke/transient ischaemic attack, myocardial infarction (MI), hospitalisation for heart failure, hospitalisation for angina pectoris, aortic dissection, lower limb arterial obstruction, emergency thrombosis, transition to dialysis, or doubling of serum creatinine levels.

The study was prematurely stopped after a median observation time of 3.27 years, because of unequivocal benefit in the valsartan group. Compared with the non-ARB arm, fewer individuals in the valsartan arm reached a primary end point. This difference was mainly attributable to a reduced incidence of angina pectoris and stroke/transient ischaemic attack (table 1).
There was also a significant reduction in one of the secondary end points, new-onset diabetes, in the valsartan group (p=0.028).
Blood pressure at baseline was 157/88 mmHg in both groups. Mean blood pressure during the treatment period was reduced to a similar extent in both groups – to 133.1/76.1 mmHg in the valsartan arm and 133.3/76.0 mmHg in the non-ARB arm.
Principal investigator Professor Hiroaki Matsubara commented: “The study showed that valsartan has the additional benefits of cardiovascular event prevention for hypertensive patients in East Asia with metabolic syndrome or high-risks.”
Discussant of the study, Professor Frank Ruschitzka (University Hospital, Zurich, Switzerland), described the results as “almost too good to be true.” But he noted that there was no effect of valsartan on MI, which weakened the observation of a significant effect on angina.
Conflicts with VALUE
He also pointed out that the Kyoto study results conflicted with those of the VALUE trial, which suggested an increased risk of MI associated with valsartan and a trend towards more strokes. A new meta-analysis of six ARB trials, including the Kyoto study, has shown a reduction in stroke with the ARBs but a trend toward an increased risk of MI when compared with active treatment, he added.
“My take-home message is that ACE inhibitors and calcium-channel blockers are my number-one choice and ARBs are for those who don’t tolerate ACE inhibitors”, Professor Ruschitzka concluded.
The Kyoto Heart Study was published online on August 31st in the European Heart Journal (doi:10.1093/eurheartj/ehp363) to coincide with presentation at the ESC. An accompanying editorial suggests that Asians may be particularly receptive to the protective effects of ARBs. It notes that this was shown in the RENAAL trial, where most of the benefits occurred in the Asian subpopulation, whereas in VALUE, less than 3.5% of participants were Asian.
ARBs are efficacious and even superior to other drug classes in stroke prevention, but their efficacy with regard to coronary events remains uncertain, the editorial concludes.
Heart rhythm problems often misdiagnosed as epilepsy
One in eight adult patients in the UK, previously thought to be suffering from epilepsy but in whom this diagnosis was in doubt, in fact had heart rhythm abnormalities commonly found in patients with syncope, results of the REVISE trial have shown.
The study included 40 patients who had experienced at least three blackouts or “transient loss of consciousness” in the previous year and on examination by a neurologist there was doubt about the diagnosis of epilepsy. They were given an implantable ECG in a low risk, 20 minute operation and followed for nine months.
Results of the ECG monitoring showed that five of the patients had an abnormality in their heart rhythm and four of these patients who underwent a pacemaker insertion were subsequently found to be free of their symptoms.
Lead investigator, Dr Sanjiv Petkar (Manchester Heart Centre) noted that previous studies have shown that up to 25% of patients thought to be suffering from epilepsy do not actually have this condition, and that many patients are taking drugs for epilepsy which they do not need. In some patients syncope can mimic epilepsy – a temporary decrease in blood supply to the brain which occurs in syncope can result in irritation of brain cells causing abnormal movements, which to a lay person can look very similar to epilepsy, he said. In the general population, syncope is much more common than epilepsy, affecting 25% of the population at any given time, more so in the elderly, he added.
First European guidelines for reducing cardiac risk in non-cardiac surgery
New guidelines issued by the European Society of Cardiology address for the first time the risk of cardiac complications in patients undergoing non-cardiac surgery.
They note that after major surgery the incidence of cardiac death varies between 0.5% and 1.5%, and the incidence of non-fatal cardiac complications range from 2.0% to 3.5%. When applied to the population in the European Union member states these figures translate into 150,000 to 250,000 life-threatening cardiac complications resulting from non-cardiac surgical procedures annually.
The guidelines recommend a practical, stepwise evaluation of the patient, which integrates cardiac risk factors and test results with the estimated stress of the planned surgical procedure. They advise preoperative assessment of clinical risk factors, such as heart failure, previous myocardial infarction, and diabetes mellitus, to stratify patients according to risk of cardiac events. But the use of additional cardiac testing, such as echocardiography or exercise testing, is only recommended for patients with multiple risk factors scheduled for high-risk surgery, in order to assess the presence and extent of ischaemic heart disease. The guidelines say that prophylactic coronary revascularisation is rarely indicated just to get the patient through surgery.
Preoperative cardiac risk evaluation also offers a unique opportunity to identify and treat risk factors. The initiation of lifestyle changes and medical therapy for cardiac risk factors should be done prior to surgery, as interventions improve both perioperative and late outcome, the guidelines state.
Other recommendations include the following:
- The cause of perioperative cardiac events are complex, the assumption that a single drug can intervene in all these factors is unlikely. A combination of beta-blockers, statins, aspirin, and angiotensin-converting enzyme inhibitors are probably the best medical option.
- A low-dose of beta-blocker, timely started prior to surgery is recommended. Beta-blocker dose should be titrated to achieve heart rate between 60 and 70 per minute.
- Statins with a long half-life time or extended release formulations are recommended, to bridge the period immediately after surgery when oral intake is not feasible.
- Aspirin therapy should be continued, and only stopped in those in which haemostasis is difficult to control during surgery.
- Preoperative coronary intervention using stents should be discussed with the treating surgeon and anaesthesiologist, as anti-platelet therapy (aspirin and/ or clopidogrel) influences perioperative management.
- A patient should live long enough to enjoy the benefits of surgery.
