The treatment of raised cholesterol has advanced significantly in the last 25 years: fibrates, statins, bile acid sequestrants, ezetimibe, and more. In October 2007, colesevelam hydrochloride was launched into the UK market. This article reviews where this product fits into everyday clinical practice, in which patients it is best suited, and discusses practical issues in the everyday use of this reformulated bile acid sequestrant.
Introduction
Bile acid sequestrants (BAS) were the first class of lipid-lowering drug to be developed for reducing blood cholesterol levels.1 Now, after their introduction 30 years ago, BAS still continue to command a position in the treatment of hyperlipidaemia.2
How do BAS work?
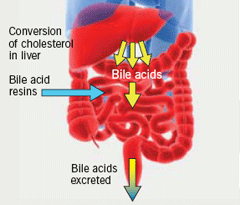
BAS bind to negatively charged bile acids in the intestine and impede their absorption (figure 1). This process depletes the bile acid pool and disrupts the enterohepatic circulation of bile acids, thus, increasing the synthesis of bile acids. Cholesterol is, therefore, diverted to bile acid synthesis, thereby reducing circulating low-density lipoprotein cholesterol (LDL-C) levels.
In spite of a significant reduction in total cholesterol (TC) and LDL-C with the administration of conventional BAS, administration of these agents proved difficult in clinical practice since they were administered as granules with limited palatability and increased gastrointestinal (GI) side effects.
What is new with BAS?
This class of lipid-regulating drug was recently modified by the addition of long hydrophobic side chains. This increased its bile acid binding capacity, increased its affinity and specificity for bile acids, and its GI tolerability. Since it is a hydrogel, it absorbs water and is not absorbed from the GI tract. This formulation was launched in the UK as colesevelam hydrochloride (Cholestagel®) in October 2007. It has been shown in numerous studies to effectively lower LDL-C3-6 with potential advantages over existing BAS.
Although colesevelam decreases TC and LDL-C, it increases triglyceride levels. Therefore, as with other BAS, colesevelam should be used with caution in patients with triglycerides >3.4 mmol/L.7
The recommended dose when used with a statin is 4–6 x 625 mg tablets daily; either two to three tablets twice daily or six tablets once daily, taken with a meal (3.75 g/day). Theoretically, the drug should be taken with a meal, since the output of bile acid is higher after a meal. It can be dosed at the same time as a statin. When used as monotherapy, the recommended starting dose is three tablets twice daily or six tablets once daily, with a meal. The maximum dose is seven tablets daily (4.375 g/day).
Colesevelam addresses many of the issues that made BAS unpopular to take.
Compliance
While compliance could traditionally be difficult with BAS due to their formulation,1 a point highlighted in a recent HEART UK survey,8 the new tablet formulation of colesevelam now makes this drug class much more palatable and convenient to take, potentially increasing compliance. A six-month clinical trial showed that compliance in patients receiving colesevelam 3.75 g/day was 88%.5 Evidence from clinical practice also supports the fact that colesevelam is generally well tolerated.
Timing of dose and drug interactions
A major issue with BAS was the difficulties in timing of the dose, since they had to be administered three hours apart from other drugs, up to four times daily.9 The once- or twice-daily dose of colesevelam2 means that patients find it easier to co-ordinate their dose with the other drugs they are often taking. The unique polymer structure confers colesevelam a lower risk for potential drug interactions,10 thus giving colesevelam a significant advantage over other previously available BAS since it means that colesevelam can be taken with other drugs, including statins,7 without the patient having to take other agents one to two hours before or four hours after their BAS. This was, at best, inconvenient and, at worst, led to discontinuation of therapy.
The bioavailability of digoxin, metoprolol, quinidine and valproic acid has been confirmed to be unaffected to any great extent when taken with colesevelam. However, there is a reduction of the area under the curve (AUC) of sustained-release verapamil with colesevelam. As with all agents, this drug’s inter-individual variability should not be forgotten.
It should also be noted that anticoagulant therapy should be monitored closely in patients on colesevelam also receiving warfarin or similar agents. BAS, including colesevelam, have been shown both to reduce absorption of vitamin K and to interfere with warfarin’s anticoagulant effect.7
Discontinuation rates
Traditional BAS have been associated with high discontinuation rates, with 34% of patients stopping treatment over a one-year period, primarily due to adverse effects.11 During a clinical study in 3,806 patients receiving long-term colestyramine, at one year, 68% reported at least one moderate-to-severe GI adverse effect (39% moderate-to-severe constipation), compared with 43% and 10%, respectively, for placebo therapy.12 Discontinuation rates with colesevelam are expected to be low in view of its reduced risk for interactions and improved side effect profile.
Adverse events
Adverse GI effects (constipation, dyspepsia and flatulence) are associated with the traditional BAS, colestipol and colestyramine.10 In clinical studies5 and in clinical practice,13 GI side effects with colesevelam are generally mild to moderate.7
Which patients?
Since its launch in the UK, colesevelam has proven to be of most value in clinical practice in two specific patient groups.
Difficult-to-treat patients, for primary or secondary prevention, e.g. familial hypercholesterolaemia (FH)
A significant number of patients with FH have very high cholesterol levels that are not lowered sufficiently using statins. An LDL-C target of 2 mmol/L for secondary prevention is often difficult to achieve with statins, even at higher doses. Use of colesevelam is particularly of value in these patients who do not reach their LDL-C target levels, and appear to respond well to colesevelam. Fibrates are not a suitable option in this high-risk group since they do not sufficiently reduce LDL-C.
Statin-intolerant patients
Statin-intolerant patients may benefit from colesevelam monotherapy. Intolerance to statins increases with increasing statin dose and age.10 Studies show that 7–9% of patients in the clinic population are intolerant to statins.14 This is often higher than that shown in clinical studies, which are obviously undertaken in a well-controlled environment.11 While these patients are suitable candidates for consideration of colesevelam, efforts should be made to confirm that patients are truly intolerant to statins, since statins remain the most effective LDL-lowering drug available.
It should be noted that non-adherence is not the same as intolerance. The rate of non-adherence to statins can be as high as 61.1% in the first year and, after three and six years, only 21.8% and 9.6% of patients, respectively, have been shown to remain on statins.15
Additional LDL-C reduction is seen when combining colesevelam with ezetimibe in patients who are intolerant to statins. The complementary mode of action of these two agents means that it is logical to use them together in difficult-to-treat patients. However, it must be noted that this is an unlicensed indication for colesevelam.16,17 Ezetimibe, a non-statin cholesterol-lowering agent, has recently been the subject of a National Institute for Health and Clinical Excellence (NICE) assessment and deemed cost-effective in high-risk patients intolerant of statins.18
LDL-C targets: current guidelines
LDL-C targets vary according to the specific guideline being followed: some suggest an LDL-C level below 2 mmol/L19 or a reduction of 30% from baseline. The recent lipid modification guideline does not recommend a target for primary prevention but the LDL-C target for secondary prevention is below 2 mmol/L. The LDL-C targets in the NICE diabetes guideline follow the Joint British Societies’ (JBS 2) recommendation of <2 mmol/L. Whatever the exact LDL-C targets, the message that ‘lower is better’ in high-risk patients is clear and, for some patients in clinical practice, this can be a challenge with statins alone or in combination with ezetimibe.
Failure to reach targets – why we need another cholesterol-lowering agent
A large number of individuals still fail to reach LDL-C targets with the current lipid-lowering drugs. This is often due to non-compliance, intolerance, inadequate dosage or side effects at higher doses. Combination therapy for hyperlipidaemia is not as popular as that for hypertension. The reasons for this are the perceived increase in the incidence of side effects, such as myositis, and issues with compliance.
In one study in patients with moderate-to-severe hypercholesterolaemia (LDL-C = 4.1–6.6 mmol/L) with multiple risk factors (e.g. CHD, atherosclerosis, diabetes), 13–28% failed to reach LDL-C goals of <2.5 or 2.6 mmol/L, despite either high-dose statin20 or statin plus ezetimibe.21 In another study, in patients with FH, 76–95% failed to reach LDL-C goals of <2.6 mmol/L, despite either high-dose statin22,23 or statin plus ezetimibe.23 Even for hypercholesterolaemic patients without established coronary heart disease (CHD), approximately 40% require >30% reductions in LDL-C to achieve National Cholesterol Education Program (NCEP) target levels. These data suggest that a significant number of patients, with or without CHD, require more intensive lipid-lowering therapy than that used in current practice.3 In our secondary/tertiary care settings, the proportion of patients referred who do not reach targets often require two or more drugs.
Overview of clinical data for colesevelam
Key clinical evidence for colesevelam comprises two placebo-controlled trials5,6 and three studies of combined treatment with a statin3,4,24 (table 1). Colesevelam has also been shown to have additive lowering effects on LDL-C levels in combination with ezetimibe16,17 and fenofibrate.25 However, these latter combinations are unlicensed indications. No comparative trials with colestyramine or colestipol have been published.26,27
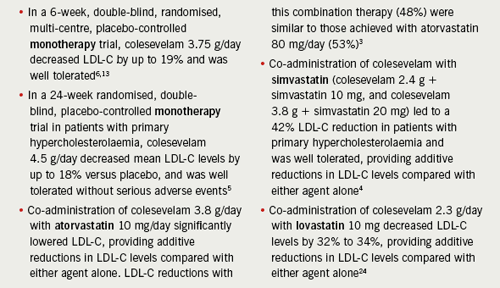
From a clinical practice perspective, it is particularly important to note the results of the Hunninghake et al. study,3 since the combination of a low-dose statin (atorvastatin 10 mg/day) plus colesevelam (3.8 g/day) achieved a similar reduction in LDL-C as high-dose statin (atorvastatin 80 mg/day) alone.
Colesevelam is not currently approved for use in children or pregnant women. However, although caution must clearly be exercised, it is this author’s view that colesevelam might be considered an option if treatment should be continued during pregnancy or when planning for pregnancy. BAS are not metabolised, and so systemic exposure of the foetus to the drug during pregnancy is unlikely.
Also of interest, colesevelam administered to people with type 2 diabetes has resulted in a decrease in blood glucose levels and glycated haemoglobin levels, shown in the glucose-lowering effect of WelChol (GLOWS) study.28
Cost issues
As always, cost must be a consideration. While there are no official cost-effectiveness data for colesevelam, e.g. from NICE, the cost of a typical one month’s treatment for the three BAS – Questran®, Colestipol® and Cholestagel® – can be seen in table 2.
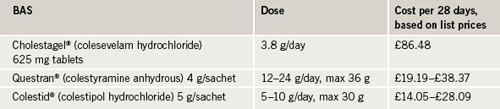
It can be seen that, in fact, colesevelam can be considered to be cost-effective compared with other BAS currently available. When considering cost, BAS should not be compared against other drug groups since, when BAS need to be used, other options are often not appropriate. From the author’s experience, replacing other BAS with colesevelam in the formulary is not seen as a problem by most hospital pharmacies because the price difference is not significant between these agents.
Summary
Low-dose combination therapy with statins and non-statins is becoming more popular due to complementary mechanisms of action, potentially improved safety profile, and improved side effect profile compared with giving high-dose statins.
Colesevelam offers us another drug in our armoury of effective LDL-C lowering agents and should be considered in those who are difficult-to-treat and/or intolerant to statins, in whom LDL-C reduction is mandatory for long-term risk
reduction.10 It is not always easy for us to achieve cholesterol targets and ensure compliance, but we must persevere with our difficult-to-treat patients using all the agents available to us.
Conflict of interest
DN has received research grants from MSD, Pfizer and Solvay. She has also received speaker’s and advisory board honoraria from Abbott Labratories, AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, MSD, Pfizer and Genzyme.
Key messages
- A large number of individuals still fail to reach LDL-C targets with the most commonly used lipid-lowering drugs
- Colesevelam is a newer bile acid sequestrant (BAS) preparation with improved gastrointestinal tolerability
- Colesevelam has been shown in numerous studies to effectively lower low-density lipoprotein cholesterol with potential advantages over existing BAS
- Patients who may benefit from colesevelam include those with familial hypercholesterolaemia and an insufficient response to statins and those intolerant to statins
References
- Steinmetz KL. Colesevelam hydrochloride. Am J Health Syst Pharm 2002;59:932–9.
- Insull W Jr. Clinical utility of bile acid sequestrants in the treatment of dyslipidemia: a scientific review. South Med J 2006;99:257–73.
- Hunninghake D, Insull W, Toth P et al. Coadministration of colesevelam hydrochloride with atorvastatin lowers LDL cholesterol additively. Atherosclerosis 2001;158:407–16.
- Knapp HH, Schrott H, Ma P et al. Efficacy and safety of combination of simvastatin and in patients with primary hypercholesterolemia. Am J Med 2001;110:352–60.
- Insull W, Toth P, Mullican W et al. Effectiveness of colesevelam hydrochloride in decreasing LDL cholesterol in patients with primary hypercholesterolemia: a 24-week randomized controlled trial. Mayo Clin Proc 2001;76:971–82.
- Davidson MH, Dillon MA, Gordon B et al. Colesevelam hydrochloride (Cholestagel). A new, potent bile acid sequestrant associated with a low incidence of gastrointestinal side effects. Arch Intern Med 1999;159:1893–900.
- Cholestagel Summary of Product Characteristics, August 2008.
- H·E·A·R·T UK Patient Survey. Hypercholesterolaemia management in the UK, August 2008.
- Questran Summary of Product Characteristics, September 2005.
- Bays H, Dujovne C. Colesevelam HCl: a non-systemic lipid-altering drug. Expert Opin Pharmacother 2003;4:779–90.
- Andrade SE, Walker AM, Gottlieb LK et al. Discontinuation of antihyperlipidemic drugs—do rates reported in clinical trials reflect rates in primary care settings? N Engl J Med 1995;332:1125–31.
- The Lipid Research Clinics Coronary Primary Prevention Trial (LRC-CPPT) results. II. The relationship of reduction in incidence of coronary heart disease to cholesterol lowering. JAMA 1984;251:351–64.
- London New Drugs Group, APC/DTC briefing document, January 2008, (Colesevelam).
- Johri N, Parra S, Mikhailidis DP et al. Survey of patients with FH in context of draft NICE guidelines. Atherosclerosis 2008;199(1).
- Vinker S, Shani M, Baevsky T et al. Adherence with statins over 8 years in a usual care setting. Am J Managed Care 2008;14:388–92.
- Bays H, Rhyne J, Abby S et al. Lipid lowering effects of colesevelam HCl in combination with ezetimibe. Curr Med Res Opin 2006;22:2191–200.
- Zema MJ. Colesevelam HCl and ezetimibe combination therapy provides effective lipid-lowering in difficult-to-treat patients with hypercholesterolemia. Am J Ther 2005;12:306–10.
- National Institute for Health and Clinical Excellence. Ezetimibe for the treatment of primary (heterozygous familial and non-familial) hypercholesterolaemia.
London: NICE, November 2007. Available from: http://www.nice.org.uk/guidance/index.jsp?action=byID&r=true&o=11709 - Joint British Societies. JBS2: guideline on prevention of cardiovascular disease in clinical practice. Heart 2005;91:1–52.
- Leiter LA, Rosenson RS, Stein E et al.; POLARIS study investigators. Efficacy and safety of rosuvastatin 40 mg versus atorvastatin 80 mg in high-risk
patients with hypercholesterolemia: results of the POLARIS study. Atherosclerosis 2007;194:e154–e164. - Feldman T, Koren M, Insull W Jr et al. Treatment of high-risk patients with ezetimibe plus simvastatin co-administration versus simvastatin alone to attain National Cholesterol Education Program Adult Treatment Panel III low-density lipoprotein cholesterol goals. Am J Cardiol 2004;93:1481–6.
- Stein EA, Strutt K, Southworth H et al.; HeFH Study Group. Comparison of rosuvastatin versus atorvastatin in patients with heterozygous familial hypercholesterolemia. Am J Cardiol 2003;92:1287–93.
- Stein E, Stender S, Mata P et al.; Ezetimibe Study Group. Achieving lipoprotein goals in patients at high risk with severe hypercholesterolemia:
efficacy and safety of ezetimibe co-administered with atorvastatin. Am Heart J 2004;148:447–55. - Davidson MH, Toth P, Weiss S et al. Low-dose combination therapy with colesevelam hydrochloride and lovastatin effectively decreases low-density lipoprotein cholesterol in patients with primary hypercholesterolemia. Clin Cardiol 2001;24:467–74.
- McKenney J, Jones M, Abby S. Safety and efficacy of colesevelam hydrochloride in combination with fenofibrate for the treatment of mixed hyperlipidemia. Curr Med Res Opin 2005;21:1403–12.
- Chaplin S, Durrington P. Colesevelam: new alternative or adjunctive treatment to statins. Prescriber 2008;19 April:27–30.
- European Medicines Agency. Scientific Discussion: Colesevelam. Available from: http://www.emea.europa.eu/humandocs/PDFs/EPAR/colesevelam/586403en6.pdf
[accessed 18/08/08]. - Zieve FJ, Kalin MF, Schwartz SL et al. Results of the Glucose-Lowering Effect of the WelChol Study (GLOWS): a randomised, double-blind, placebo-controlled pilot study evaluating the effect of hydrochloride on glycaemic control in subjects with type 2 diabetes. Clin Therapeut 2007;29:74–83.
- British National Formulary. Number 55, September 2008.
