Chronic kidney disease (CKD) has been redefined by the American National Kidney Foundation with stages based on the estimated glomerular filtration rate (eGFR) or other evidence of kidney abnormality.(1) Five stages were originally described by the Kidney Disease Outcomes and Quality initiative.
Introduction

eGFR is calculated from serum creatinine levels using the MDRD equation which was developed during the Modification of Diet in Renal Disease Study.2 This calculation is most reliable when kidney function is significantly abnormal. Values above 60 ml/min/1.73m² should not be used to define stages 1 or 2 CKD without other evidence of kidney abnormality, such as an abnormal scan or abnormalities of urinalysis.3
Using this classification it is estimated that in the National Health and Nutrition Examination Survey (NHANES) III study of an American population aged 20 years and over from 1988–1994, the prevalence of CKD was over 10%, and that of the clinically important stages 3–5 was 4.9%.4 More recent NHANES data from 2003–2006 suggests the prevalence of those with an eGFR <60 ml/min is 8.1%.5 Estimates from the UK Quality Outcomes Framework (QOF) primary care databases in 2008/9 suggest a lower UK prevalence of 4.1%,6 although the New Opportunities for Early Renal Intervention by Computerised Assessment (NEOERICA) study suggests a prevalence of nearer 10%.7
The early detection of CKD is important because it gives the opportunity to reduce the progression to end-stage kidney disease, allows the patient to be involved in choices about their treatment and ultimately may improve their life expectancy. A study of one county in Norway followed over 65,000 people with CKD stages 3–5 (an eGFR
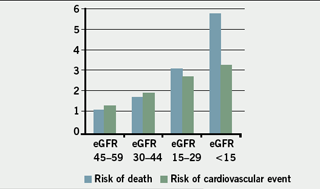
<60 ml/min/1.73m²) for eight years. Although 51 progressed to end-stage renal disease, there were 506 cardiovascular deaths.8 A patient with early CKD stage 3–4 is more likely to die of a cardiovascular event than require dialysis, however, when eGFR falls below 15 ml/min/m² the risk of a cardiovascular event is very high but equivalent to the risk of progressing to renal replacement therapy.
 The important areas of management of people with CKD for a generalist are:
The important areas of management of people with CKD for a generalist are:
- To identify people at risk and perform appropriate tests
- To establish the diagnosis and degree of kidney damage
- To commence evidence-based treatments to delay progression
- To manage and assess cardiovascular disease (CVD) risk
- To establish those patients who require referral and arrange timely referral to a renal specialist.
1. Decide who should be tested for CKD
The majority of patients with stages 3–5 CKD will have diabetes, hypertension, and evidence of established CVD. All of these patients should have regular kidney function tests included in the annual review of their condition in primary care. Patients with heart failure and those taking angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) are particularly at risk of CKD.

People from South Asia and African-Caribbean ethnic groups are at higher risk,5 although this is, of course, partially linked to their increased risk of metabolic syndrome and hypertension.
People with a history of urinary tract abnormalities, including recurrent urinary tract infections, stones and bladder outlet obstruction, should also be assessed.
A comprehensive list is shown in table 1.
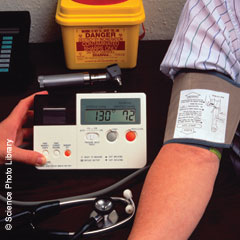
2. Carry out an appropriate test of kidney function: eGFR
eGFR should now be reported with serum creatinine results by all laboratories. Serum creatinine may be elevated after a cooked meat meal and in dehydration. Ideally, the blood test should be performed without eating meat for 12 hours but with the patient well hydrated. It is also important to correct for ethnicity by multiplying calculated eGFR by 1.21 for people of African-Caribbean or African ethnicity due to larger muscle mass. Values of >60 ml/min/1.73m² may simply be reported as that because of inaccuracy of the estimate above this level.
eGFR should not be used to estimate kidney function in those under 18 years, pregnant women and those with extremes of muscle mass.
Where eGFR is found to be <60 ml/min/1.73m² for the first time it is important to repeat the test within two weeks and even sooner if the patient felt ill, if urinalysis showed blood and protein, or if there was any evidence of a systemic disease, e.g. vasculitic rash, to ensure that the person does not have acute kidney injury.3 In many cases, however, there will be previous creatinine levels for comparison to assess decline in kidney function and, if required, the eGFR can be estimated using one of the online eGFR calculators, on the British Renal Society website for example. The calculated result may differ slightly from that provided by the local laboratory, which will use a correction factor related to the creatinine assay used, but the value may enable you to assess progression.
In order to confirm the diagnosis of CKD there should be at least two abnormal results over three months apart.
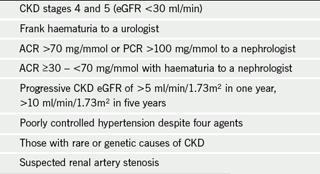
National Institute for Health and Clinical Excellence (NICE) guidelines recommend that those people who have an eGFR persistently below 30 ml/min/1.73m², i.e. stages 4 and 5 CKD should be considered for referral.3
3. Test the patient’s urine
All people newly diagnosed with CKD should have a urine dipstick test for blood and protein. In general, older people with frank haematuria should be referred to a urologist, although a young person with visible haematuria is likely to have IgA nephropathy. Those with dipstick-positive protein and microscopic haematuria (without evidence of urinary infection) should be referred to a nephrologist. It is important to check for infection and red cells in a mid-stream urine (MSU) test at this stage before considering referral.
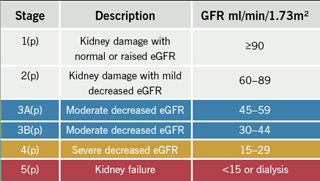
It is important to quantify proteinuria using albumin creatinine ratio (ACR) where possible, although where there is dipstick-positive proteinuria then protein creatinine ratio (PCR), may be adequate. ACR is a more sensitive test for small amounts of leaked protein (microalbuminuria) and this is, therefore, a vital part in the management of people with diabetes where it indicates the earliest stage in the development of diabetic nephropathy. Although it is considered to reflect higher cardiovascular risk, its significance in people who do not have diabetes is less clear. In people who do not have diabetes an ACR of ≥30 mg/mmol or PCR of ≥50 mg/mmol is classified as significant proteinuria when the result is confirmed on a second urine sample. The suffix p can then be added to their CKD staging identifying their additional risk of complications of their CKD (table 2). People with an ACR of ≥70 mg/mmol or PCR ≥100 mg/mmol should be considered for specialist referral.3
4. Carry out appropriate history taking and examination
It is important to establish if there is a history of familial CKD. Has a relative had stage 5 kidney disease, and, particularly, is there a history of autosomal dominant polycystic kidney disease?
Is there a history of multi-system connective tissue disorder, such as systemic lupus erythematosus (SLE), which may damage the kidneys? Are there any urinary tract symptoms, in particular, those of bladder outlet obstruction?
Take a thorough history of prescribed, over-the-counter and herbal medications. The worst culprits are the non-steroidal anti-inflammatory drugs (NSAIDs), but drugs such as cytotoxics, lithium, gold and cyclosporin are important. Trimethoprim may raise creatinine levels in vulnerable individuals. Chinese herbal medicines and foraged mushrooms may also be nephrotoxic.
Undertake a full physical examination. Look for signs of anaemia, which becomes more common if eGFR is <30 ml/min in people without diabetes, but is more widespread in people with diabetes where it can develop at stage 3B CKD.
Check the pulses for signs of peripheral vascular disease (PVD) and the blood pressure, which is an important factor both as a cause and drive for progression.
Check the abdomen for renal masses, and also for a palpable bladder to check for the important reversible cause of CKD – bladder outlet obstruction.
5. Assess the patient’s cardiovascular risk
People with CKD are at increased risk of CVD. An estimate of risk using a risk prediction tool should be made; however, there are limitations of the commonly used prediction tools. The estimate of risk using the Joint British Societies (JBS2) Framingham-based calculation,9 only includes CKD where there is proteinuria; at this stage it is considered to reflect target organ damage and, thus, a CVD risk of >20%. In QRISK2, CKD is included simply as ‘yes’ or ‘no’ in the calculation, and this may underestimate the very high risk in those with CKD stage 5 and overestimate the risk in an individual with an eGFR of 55–59 ml/min. In people with diabetes, the presence of microalbuminuria is a strong predictor of increased CVD risk, however, this is not included in the UK Prospective Diabetes Study (UKPDS) Risk Engine version 2.0. We are awaiting the results of large primary care cohort studies, such as the Renal Risk In Derby (R²ID), to clarify CVD risk in people with CKD.
Until the results become available for people with non-diabetic CKD, it may be wise to consider the results of a study by Go et al., which showed that cardiovascular risk significantly increases when eGFR falls below 45 ml/min, i.e. CKD stage 3B, and to optimise management in these patients.10
NICE guidelines recommend the treatment of hyperlipidaemia according to cardiovascular risk, however, not all statins are suitable for patients with CKD, particularly when eGFR falls below 30 ml/min.
6. Give the patient appropriate information and lifestyle advice
At this stage the diagnosis of CKD will have been confirmed, and it is important to discuss the impact of this for the patient. This will largely be driven by their age and the severity of their CKD. An elderly patient with a stable creatinine level and an eGFR of 58 ml/min is unlikely to develop complications of CKD. However, practitioners need to consider renal function when making prescribing choices and patients should have regular monitoring of kidney function.
Younger patients with evidence of progression will need counselling about the risks of their CKD, and patient information should be made available to them, along with links to excellent websites. However, it should be made clear that some of these focus on preparation for renal replacement therapy, which may not be relevant and can be frightening for the patient.
Advice about smoking cessation, weight control and salt restriction are very important for people with CKD. Obesity is associated with progression of CKD and some people have salt-sensitive hypertension.
7. Control the patient’s blood pressure

Blood pressure management is the cornerstone of the management of CKD, and reliable blood pressure recording is so important. It may be helpful to obtain ambulatory readings to assess blood pressure control. NICE offers clear guidance on the choice of medication and the targets for treatment are a systolic target range of 120–139 mmHg and diastolic below 90 mmHg, which is lower than for any other condition. This is even more stringent for people with diabetes and CKD or proteinuria where the aim is to keep the systolic between 120 and 129 mmHg and diastolic below 80 mmHg.3
Failure to reduce blood pressure to recommended targets using four agents in maximally tolerated doses is considered to require a referral for a specialist opinion.3
Those people in whom a secondary cause, other than CKD, for their high blood pressure is considered should also be referred.
It is important to remember that when starting an A drug (ACE inhibitor or ARB) in people with CKD, creatinine is likely to rise and that hyperkalaemia may be an issue. Guidelines recommend that creatinine and potassium should be checked before starting or increasing the dose of an A drug, and one to two weeks after each dose change. A rise in creatinine of >30% without other causes of deterioration in renal function, volume depletion for example, should trigger discontinuing the drug, and consideration of underlying renal artery stenosis which can be an indication for referral.
8. Make an assessment for endocrine complications of CKD
Endocrine complications become more prevalent in CKD stages 4 and 5. Early guidance suggested that all people with CKD should have an assessment of calcium and phosphate metabolism, but this is now only recommended when eGFR <30 ml/min/1.73m², and expensive testing for parathyroid hormone and vitamin D should be avoided in primary care.
Anaemia (Hb <10.5 g/dl), is a significant problem in people with CKD 4 and 5. In people with diabetes and CKD it may occur when eGFR falls below 45 ml/min and all of these patients should have a blood count as part of their review. It is important to check for other causes of anaemia, such as iron deficiency, before assuming that patients have anaemia related to their CKD, and these results should be included with any referral.
9. Assess progression of renal dysfunction
The majority of patients with early CKD will have hypertension, diabetes mellitus or cardiovascular disease. Although there may not be many eGFR results in the primary care records, it is likely that there will be many creatinine values from previous reviews. Creatinine alone is not a good indicator of progression in the early stages of renal disease; however, previous results if included with a referral will give an indication of the progress of the kidney disease. Results of previous urine tests will also be helpful.
Progression is defined in NICE guidelines as a decline in eGFR of >5 ml/min/1.73m² within one year, or >10 ml/min/1.73m² within five years. Patients with this degree of progression should be considered for referral, particularly when projected progression suggests that renal replacement therapy will be required in the future.
The guidelines also suggest that a renal ultrasound scan should be undertaken in people with progressive CKD, particularly where there are symptoms of urinary tract obstruction, haematuria, or stage 4 or 5 CKD, and this may facilitate referral, but should not delay it.
10. Refer appropriately
When referral is considered appropriate it is important to also consider an individual’s co-morbidities and wishes concerning their long-term care. Many centres offer alternative pathways in which general practitioners can access specialist advice, as opposed to the patient visiting a renal out-patient department, and a discussion may be more appropriate. For those involved in developing referral pathways there is a CKD pathway on the Department of Health 18-week website.11
A summary of NICE guidelines for referral is shown in table 3.
A referral should include the following information (this may result in an early letter of advice from the nephrologist, rather than an unnecessary out-patient appointment):
- Results of previous eGFR and creatinine measurements, with dates (particularly important if referral hospital is not where blood samples are analysed)
- Appropriate medical and drug history
- Blood pressure measurements over time
- Urine results with ACR and dipstick testing
- Full blood count, calcium, phosphate, albumin and bicarbonate.
It may be important to reassure the patient that referral to a renal physician does not inevitably mean that they may need dialysis. Early intervention may stabilise their renal disease. In those who do progress, pre-emptive transplant from a living donor offers the patient the benefits of avoiding dialysis and better long-term survival.
Patients may also receive symptomatic improvement from management of renal anaemia. In addition, active conservative care provided by the multi-professional renal team may be an effective option, especially for those with extensive co-morbidities and the very elderly.
Conflict of interest
None declared.
References
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Am J Kidney Dis 2002;43(suppl 1):S1–S290.
- Levey AS, Bosch JP, Lewis JB et al. A more accurate method to estimate glomerular filtration rate from serum creatinine; a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999;130:461–70.
- National Institute for Health and Clinical Excellence. NICE clinical guideline 73. Chronic kidney disease. London: NICE, 2008.
- Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 2003;41:1–12.
- Coresh J, Selvin E, Stevens LA et al. Prevalence of chronic kidney disease in the United States. JAMA 2007;298:2038–47.
- The information centre for health and social care. QOF Acheivement and Prevalence Bulletin 2008-09. 2009. (www.ic.nhs.uk)
- Stevens PE, O’Donoghue DJ, de Lusignan S et al. Chronic kidney disease management in the United Kingdom: NEOERICA project results. Kidney Int 2007;72:92–9.
- Hallan SI, Dahl K, Olen CM et al. Screening strategies for chronic kidney disease in the general population: follow-up of cross sectional health survey. BMJ 2006;333:1047–50.
- Joint British Societies. JBS 2: Joint British Societies guidelines on prevention of cardiovascular disease in clinical practice. Heart 2005;91 (suppl V):v1–v52.
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalisation. N Engl J Med 2004;351:1296–305.
- 18 week NHS. Chronic kidney disease (CKD) renal commissioning pathways. Delivering the 18 week patient pathway. (www.18weeks.nhs.uk)
