National Health Service (NHS) costs in England grew from about £40 billion in the year 2000 to £100 billion today. That is, they have approximately doubled in real terms within a decade. However, the current economic climate in the UK has led to increasing cost awareness in the NHS. NHS managers have been charged with making £15–20 billion efficiency savings by 2015.1 Although the health service will not lose funding, GPs are under pressure to prescribe low-cost generic medicines wherever possible.2
This brief paper considers how such cost pressures may affect the use of angiotensin receptor blockers (ARBs) in the NHS, given that although losartan is the first drug in this class to become generic in March 2010, others will quickly follow suit. Valsartan loses patent protection in 2011, with candesartan and irbesartan following in 2012.
Indications for ARB use
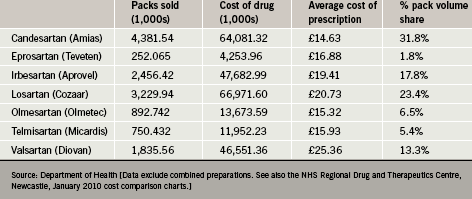
Recent data indicate that candesartan is presently the most widely used ARB in England, accounting for almost a third of all ARB prescriptions. This is in part because it has been competitively priced compared with other ARBs. In average prescription cost terms, candesartan has in recent years enjoyed an approximate 25% price advantage over its main high-volume competitor (table 1).
At present, there are variations with respect to the licensed indications for ARBs in the UK. All drugs in the class are licensed for the treatment of hypertension whereas only three (candesartan, losartan and valsartan) are indicated for chronic heart failure. Valsartan alone is licensed for use post-myocardial infarction (MI) in patients with left ventricular systolic dysfunction (LVSD). Irbesartan and losartan are the only ARBs presently approved for the treatment of nephropathy in patients
with type 2 diabetes mellitus.
However, it is often assumed that all ARBs are equivalent. If this is believed uncritically, then NHS pharmaceutical advisers and economists may be influenced exclusively by price considerations. The advent of generic level price competition ‘class effect’ prescribing, such that all drugs within a class are assumed to have identical safety and efficacy profiles,3 in some instances could undermine public interests in both continuity of care and the optimisation of individual and/or population level health outcomes.
Broader implications of changing ARB presciptions
Usher-Smith has highlighted the challenges involved in switching patients’ medicines for purely cost-saving purposes.2, 4 He reported his experience within a PCT that encouraged switching established patients from losartan to candesartan in 2005–2006 on cost grounds. Such a policy had originally been estimated to generate a national three-year saving of £128 million. However, in August 2007, the price of losartan was decreased, resulting in reduced actual annual savings. This illustrates the point that the outcomes of cost-reducing measures may be dependent upon unpredictable market adjustments that can undermine their cost-effectiveness even though in this particular instance a significant drop in blood pressure was seen after patients switched to candesartan. This could have been a drug-related effect, or alternatively the effect of enhanced case management associated with the effort of supporting switching. In general, it is the case that moving patients who are well established on one medicine to another demands not only increased clinician time, but also risks undermining their confidence in their clinician and reducing adherence rates. Further, if the drugs are not fully interchangeable this may have additional consequences on outcomes and (in time) overall care/service costs.
In this context, the ‘Real-Life’ study5 discussed by Meredith in this supplement looked retrospectively at candesartan and losartan use in primary care centres in Sweden. It found there was no difference in blood pressure (BP) reduction when comparing the losartan and candesartan groups (although more patients in the losartan arm required a thiazide to achieve the same BP reduction), but that candesartan use was associated with a significantly lower risk of cardiovascular events compared with losartan. The results published to date do not make it possible to quantify the reduced economic burden associated with candesartan use in this context. However, they do raise the possibility that short-term financial gains associated with a general switch to losartan therapy before other ARBs lose patent protection might be offset by factors linked, say, to reduced 24-hour BP control.
Conclusions
Making the best possible use of health resources is an important end. Yet factors other than price advantage alone should be considered before accepting policy decisions to switch patients automatically from one therapeutic agent to another. Care should be taken not to ignore clinically significant utility variations within the ARBs; since these drugs are both effective and relatively free from side effects, they may become more widely used in future as prices fall across the board.
Research such as that by Usher-Smith2 and Kjeldsen et al. 5 indicates that the routine ‘switching’ of patients from one drug to another, based on short-term unit price differences alone, might prove counter-productive. Such policies are only defensible when there is robust evidence both that the switch is beneficial and that it justifies the time and effort needed to support patients adequately during the transition from a familiar medicine to a new one. Switching should be backed by evidence regarding relevant population health outcomes
relative to the overall costs incurred and the financial resources released for alternative use.
Conflict of interest
DGT received a fee from Takeda for his involvement in this project, which was shared with a postgraduate student who kindly provided background analysis. MD received an honorarium from Takeda for his contribution to this supplement.
Key messages
- The NHS is under pressure to contain costs through the prescribing and supply of low-cost generic medicines whenever appropriate
- Losartan is the first angiotensin receptor blocker (ARB) to come off-patent (in March 2010). This may prompt some primary care trusts (PCTs) and practices to switch patients who are currently taking other ARBs to generic losartan
- There is evidence that different ARBs have pharmacologically distinct actions, which may differentially affect patient outcomes. Switching patients may not reduce financial costs as much as initially anticipated due to additional clinician time and effort needed to manage patients and because, from an economic perspective, long-term health outcomes may be impaired
- A patient-centred, evidence-based approach is needed, rather than one uncritically focused on unit drug costs.
References
- NHS 2010–2015: from good to great. Preventative, people-centred, productive. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_109876
- Usher-Smith JA, Ramsbottom T, Pearmain H, Kirby M. Evaluation of the cost savings and clinical outcomes of switching patients from atorvastatin to simvastatin and losartan to candesartan in a primary care setting. Int J Clin Pract 2007;61(1):15–23.
- Meredith PA, Murray LS, McInnes GT. Comparison of the efficacy of candesartan and losartan: a meta-analysis of trials in the treatment of hypertension. J Hum Hypertens 2009;1–7. Advance online publication, 17 December 2009; doi:10.1038/jhh.2009.99.
- Usher-Smith JA, Ramsbottom T, Pearmain H, Kirby M. Evaluation of the clinical outcomes of switching patients from atorvastatin to simvastatin and losartan to candesartan in a primary care setting: 2 years on. Int J Clin Pract 2008;62(3):480–4.
- Kjeldsen SE, Stålhammar J, Hasvold P, Bodegard J, Olsson U, Russell D. Effects of losartan vs. candesartan in reducing cardiovascular events in the primary treatment of hypertension. J Hum Hypertens 2009. Advance online publication, 5 November 2009; doi:10.1038/jhh.2009.77.
