Sulphonylureas are well established in the treatment of type 2 diabetes mellitus. They are effective in improving glycaemic control and preventing microvascular complications. Side effects that can restrict use include hypoglycaemia and weight gain. Although there is no clear evidence for reduction of cardiovascular disease from randomised-controlled trials, follow-up data from the United Kingdom Prospective Diabetes Study (UKPDS) shows reduced cardiovascular risk. Concerns about sulphonylureas causing inhibition of ischaemic preconditioning are relevant in primary angioplasty, but there is a lack of clear evidence, with a need for randomised-controlled trials to investigate this further.
Introduction

The increasing prevalence of type 2 diabetes has implications for most health professionals, with one aspect of care being efforts to improve glycaemic control. Sulphonylureas are well established for the treatment of hyperglycaemia with evidence for their effect on reducing microvascular complications. However, as for most agents used to improve glycaemic control in patients with type 2 diabetes, there is no clear evidence of macrovascular or mortality benefit. The University Group Diabetes Program (UGDP) study raised concerns about cardiovascular outcomes with sulphonylureas.1 Tolbutamide, a first-generation sulphonylurea, was tested and an excess of cardiovascular mortality was seen for the tolbutamide-treated group. There were major criticisms of the study design, including the presence of non-diabetic patients, poor compliance and ineffective randomisation.1 Subsequent evidence from the UK Prospective Diabetes Study (UKPDS) has been reassuring, confirming the safety and possible cardiovascular benefits of sulphonylureas.2 This has resulted in sulphonylureas being widely used as first- or second-line therapy in type 2 diabetes.
Pharmacology
The hypoglycaemic effect of sulphonylureas is due to enhancement of insulin secretion by pancreatic beta cells, with the mechanism outlined in figure 1. They act on the membranes of beta cells, causing closure of adenosine triphosphate (ATP)-sensitive potassium channels. They act on sulphonylurea receptors closely associated with the potassium channels. When the potassium channels are open the resting potential is lower than that required to allow voltage-gated activation of calcium channels. When the potassium channels are closed it results in opening of calcium channels and exocytosis of insulin. When glucose enters the cell, glycolytic production of ATP is increased. The rise in ATP causes closure of the ATP-sensitive potassium channels, leading to the release of insulin.
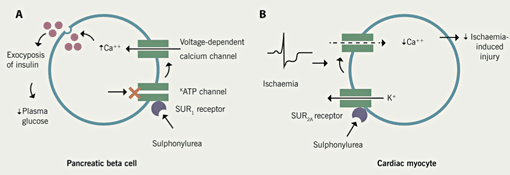
Cardiac myocytes have ATP-sensitive potassium channels associated with different forms of sulphonylurea receptor, which have differing affinities for sulphonylurea drugs (SUR1 on beta cells and SUR2A on cardiac muscle cells). This is relevant when considering possible detrimental effects of sulphonylureas during myocardial ischaemia (discussed below).
The most common and most serious side effect of sulphonylureas is hypoglycaemia. Prolonged hypoglycaemia is a greater risk with the longer-acting sulphonylureas, such as glibenclamide or modified-release gliclazide. Patients should be educated in prevention and recognition of hypoglycaemia at initiation of therapy. Specific risk factors include excessive alcohol and irregular eating patterns. As symptoms of hypoglycaemia can be non-specific, capillary blood glucose monitoring should be used to confirm. Severe episodes requiring assistance occurred in approximately 1% of the patients per year using sulphonylureas in UKPDS.2 Patients suffering a severe episode should be admitted to hospital for a period of observation. As sulphonylureas can accumulate, especially with renal impairment, prolonged glucose infusion may be required. There is evidence that the modified-release gliclazide preparation causes fewer hypoglycaemic episodes than other once-a-day preparations. In the Glucose Control in Type 2 Diabetes: Diamicron MR Versus Glimepiride (GUIDE) study, 845 type 2 diabetes patients were randomised to gliclazide modified-release (m/r) or glimepiride. HbA1c reduction was not significantly different between the two agents: 8.4% to 7.2% for gliclazide m/r and 8.2% to 7.2% for glimepiride (non-inferiority test p<0.0001). However, the rate of clinically confirmed hypoglycaemia was significantly lower for gliclazide m/r: 3.7% versus 8.9% (p=0.03).3
Weight gain is another unwanted side effect of sulphonylureas, especially in patients who are, more often than not, already obese. This is due to the effect of increased insulin secretion and decreased glucose loss in the urine. On average, patients gain roughly 1–4 kg, which plateaus at six months.
Trials of safety and efficacy
Evidence for improved glycaemic control/microvascular benefit
Sulphonylureas reduce fasting plasma glucose by an average of 2–4 mmol/L and HbA1c by 1–2%. Sulphonylureas are dependent on functioning beta cells. The natural history of type 2 diabetes typically results in progressive beta cell failure. Therefore, increasing doses will be required, and eventual treatment ‘failure’ should be expected and monitored for accordingly. The efficacy of sulphonylureas is optimised by early use to maximise the preserved beta cell function.
In UKPDS, efficacy of sulphonylureas was compared with insulin and diet alone in 3,867 newly diagnosed type 2 diabetes patients. After three years, the HbA1c values for chlorpropamide, glibenclamide, insulin and diet groups were 6.8%, 6.9%, 7.0% and 7.6%, respectively (p<0.001). At initiation of therapy the mean HbA1c was 7.2%. After 10 years, the HbA1c in the intensive group (sulphonylurea or insulin) was 7.0% compared with 7.9% in the conventional (diet alone) group (p<0.001). Over the same period, the risk of any diabetes-related end point was 11% lower in the intensive treatment group (p=0.029). Most of the risk reduction was due to a 25% reduction in the risk of microvascular end points (renal failure, death from renal failure, retinal photocoagulation, or vitreous haemorrhage) (p=0.0099).2
The Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) study looked at outcomes in 11,140 diabetic patients randomised to intensive control with HbA1c target <6.5% or standard therapy as per local guidelines. The intensive group’s primary therapy was gliclazide and the standard group were prohibited from gliclazide use. Median HbA1c achieved in the intensive group was 6.3% compared with 7.0% in the standard arm. There was a significant reduction in microvascular outcomes (hazard ratio [HR] 0.86, 95% confidence interval [CI] 0.77–0.97, p=0.01) in the intensive group.4
Evidence for cardiovascular benefit
Following UGDP, the only other prospective randomised trial looking at sulphonylurea use and cardiovascular mortality has been UKPDS.2 A total of 3,867 newly diagnosed type 2 diabetes patients were randomised to receive sulphonylurea, insulin or diet alone (conventional treatment at the time). In the 615 patients treated with glibenclamide for an average of 11 years, there was a non-significant trend to reduction in myocardial infarction compared with the control (diet alone) group: 14.1 per 1,000 patient-years versus 17.9 per 1,000 patient-years (p=0.056). Recently, a post-trial follow-up of the UKPDS population has been published: 3,277 patients were followed for six years, either through direct follow-up or questionnaire completion. There was no attempt to keep them on their trial-allocated treatment. After a year, differences in HbA1c between the groups were lost. The intensive group (taking sulphonylurea or insulin during the trial) had a new significant reduction in death by any cause (odds ratio 13%, p=0.007), and in myocardial infarction (odds ratio 15%, p=0.01).5
In the ADVANCE study, there was no significant reduction in the macrovascular outcome (HR 0.94, 95% CI 0.84–1.06, p=0.32). However, there was no increase in overall or cardiovascular mortality in the intensive arm compared with the standard control arm.4
Sulphonylureas and ischaemic preconditioning
As outlined, the mechanism of sulphonylurea effect in pancreatic beta cells is closure of ATP-sensitive potassium channels. In myocardial cells, opening ATP-sensitive potassium channels helps protect the heart during myocardial ischaemia. The reduction in voltage-dependent calcium influx reduces myocardial contractility and oxygen demand. This mechanism is believed to be important in ischaemic preconditioning, which is the phenomenon of protection of the myocardium from brief periods of ischaemia before a prolonged infarction. Therefore, there is a theoretical risk of sulphonylureas with affinity for myocardial-cell receptors inhibiting this process.
Animal models
Murry et al. first studied ischaemic preconditioning in 1986. They tested the hypothesis that multiple brief ischaemic episodes protect the heart from a sustained ischaemic insult. They compared a group of ‘preconditioned’ dogs, who had four five-minute circumflex occlusions, followed by a 40-minute occlusion, with a group who had the 40-minute occlusion with no ‘preconditioning’. The preconditioned group had a 25% (p<0.001) smaller infarct size than the controls.6
These findings have been confirmed in other animal studies. Schott et al. compared nine ‘preconditioned’ (two cycles of 10 minutes of left anterior descending artery [LAD] occlusion followed by 30 minutes of reperfusion) swine myocardia with six controls after 60-minute LAD occlusion. Infarct size was 10.4% of the risk region in the preconditioned animals compared with 48.0% in the controls (p<0.005).7
The same group studied the effect of glibenclamide on ischaemic preconditioning in rabbits. They compared infarct size in 10 rabbits that had glibenclamide administered before ischaemic preconditioning, with nine that did not receive glibenclamide. The size of infarct, as percentage of risk zone, was significantly higher in the glibenclamide group: 38.3% versus 7.3% (p<0.05).8
Mei et al. produced similar results in dogs. The size of infarct in the group given glibenclamide was 31% versus 9% in the control group (p<0.05).9
Evidence in humans
Speechly-Dick et al. looked for evidence of ischaemic preconditioning and ATP-sensitive potassium channel involvement in human tissue in 1995. They prepared right atrial samples via nine different protocols to compare the effects of a variety of factors including ATP-sensitive potassium channels and the effect of glibenclamide. They simulated ischaemia and measured percentage recovery of contractile function. The effect of preconditioning was significantly impaired by glibenclamide; 63.5% reduced to 24.8% recovery of contractile function.10
Cleveland et al. looked at the hypothesis that myocardium from patients with diabetes on long-term sulphonylureas would be resistant to the protective effects of ischaemic preconditioning. They used right atrial trabeculae obtained from patients undergoing coronary artery surgery. They compared patients who had been on long-term oral hypoglycaemics (six on glibenclamide and one using glipizide) with patients on long-term insulin, and with controls on neither. The tissue from the treatment groups was preconditioned with five minutes of ischaemia. One set of the controls was preconditioned and the rest were not. The outcomes were based on recovery of developed force, a measure of muscular contractile force. The non-preconditioned controls’ recovery of force was 28%. Preconditioned controls had significantly higher recovery at 52%. The insulin-treated group, also preconditioned, had comparable values to the preconditioned controls at 45%. The sulphonylurea group had significantly lower recovery at 27%, which is very similar to the non-preconditioned controls. The authors concluded that long-term oral hypoglycaemic use blocks theprotection due to preconditioning.11
Inhibition of ischaemic preconditioning is especially relevant during percutaneous intervention. Tomai et al. looked at 20 patients undergoing one-vessel coronary angioplasty. They received glibenclamide 10 mg (and a glucose infusion) or placebo (and a saline infusion) 90 minutes before angioplasty. ST changes on electrocardiogram (ECG) and cardiac pain scores were measured at the end of two two-minute balloon inflations. In the treatment group, the ST changes during the consecutive balloon inflations were similar; 20 mm in the first versus 23 mm in the second. Cardiac pain was greater during the second inflation; 55/100 versus 43/100 (p<0.05). In contrast, the placebo group had significantly less ST change during the second inflation; 9 mm versus 23 mm (p<0.001). The severity of pain was also lower during the second inflation; 15/100 versus 42/100 (p<0.01). They concluded that ischaemic preconditioning was abolished by glibenclamide.12
O’Keefe et al. retrospectively compared long-term outcome in 1,938 patients with type 2 diabetes who had coronary artery bypass grafting (CABG) or angioplasty for revascularisation. Ten-year survival was similar when both treatment modalities were compared in diet-treated (84% vs. 81%, respectively), and insulin-treated patients (63% vs. 64%, respectively). However, those treated with a sulphonylurea had significantly worse 10-year survival if treated with angioplasty (76% vs. 62%, respectively).13
Ischaemic preconditioning is also relevant in the context of acute coronary syndrome, when it should be protective. However, Danchin et al. looked at post-myocardial infarction hospital mortality in 487 patients with diabetes, of whom 215 were taking sulphonylureas, and there was no increased mortality. In actual fact it was significantly lower in those receiving sulphonylureas; 10.2% versus 16.9% (p=0.035).14
Discussion
Sulphonylureas are widely accepted as first- or second-line treatment in type 2 diabetes, especially in those who have lost weight or are suffering osmotic symptoms. Unwanted side effects of weight gain and risk of hypoglycaemia are to be expected. Evidence for reduction in microvascular risk is well established. Evidence regarding long-term macrovascular outcomes is less conclusive, although the long-term follow-up of the UKPDS group is positive. For cardiology patients there is a specific concern related to ischaemic preconditioning and percutaneous intervention, but there is a lack of randomised-controlled trial data looking at this area. In the interim, it is worth considering withholding sulphonylureas, if practical, in patients undergoing percutaneous coronary intervention. If alternative therapy is needed to maintain glycaemia, insulin is the most suitable choice in the short term.
Conflict of interest
None declared.
Key messages
- Well-established efficacy in controlling hyperglycaemia in type 2 diabetes
- Evidence of long-term prevention of microvascular complications of type 2 diabetes
- No clear evidence for prevention of macrovascular disease
- Possible risk of impaired ischaemic preconditioning. Suggest withholding in patients going for percutaneous coronary intervention
References
- Goldner MG, Knatterud GL, Prout TE. Effects of hypoglycaemic agents on vascular complications in patients with adult-onset diabetes. Clinical implications of UGDP results. JAMA 1971;218:1400–10.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–52.
- Schernthaner G, Grimaldi A, Di Mario U et al. GUIDE study: double-blind comparison of once-daily gliclazide MR and glimepiride in type 2 diabetic patients. Eur J Clin Invest 2004;34:535–42.
- Patel A, MacMahon S, Chalmers J et al.; on behalf of the ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–72.
- Holman RR, Paul SK, Bethel MA et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–89.
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischaemia: a delay of lethal cell injury in ischaemic myocardium. Circulation 1986;74:1124–36.
- Schott RJ, Rohmann S, Braun ER et al. Ischemic preconditioning reduces infarct size in swine myocardium. Circ Res 1990;66:1133–42.
- Munch-Ellingsen J, Bugge E, Ytrehus K. Blockade of the KATP-channel by glibenclamide aggravates ischaemic injury, and counteracts ischaemic preconditioning. Basic Res Cardiol 1996;91:382–8.
- Mei DA, Gross GJ. Evidence for the involvement of the ATP-sensitive potassium channel in a novel model of hypoxic preconditioning in dogs. Cardiovasc Res 1995;30:222–30.
- Speechly-Dick ME, Grover GJ, Yellon DM. Does ischemic preconditioning in the human involve protein kinase C and the ATP-dependent K+ channel? Circ Res 1995;77:1030–5.
- Cleveland JC, Meldrum DR, Cain BS et al. Oral sulfonylurea hypoglycemic agents prevent ischemic preconditioning in human myocardium. Circulation 1997;96:29–32.
- Tomai F, Crea F, Gaspardone A et al. Ischaemic preconditioning during coronary angiopasty is prevented by glibenclamide, a selective ATP-sensitive K+ channel blocker. Circulation 1994;90:700–05.
- O’Keefe JH, Blackstone EH, Sergeant P et al. The optimal mode of coronary revascularisation for diabetics. A risk-adjusted long-term study comparing coronary angioplasty and coronary bypass surgery. Eur Heart J 1998;19:1601–03.
- Danchin N, Charpentier G, Ledru F et al. Role of previous treatment with sulphonylureas in diabetic patients with acute myocardial infarction: results from a nationwide French registry. Diabetes Metab Res Rev 2005;21:143–9.
