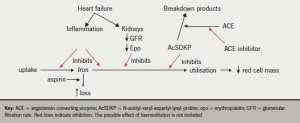
We have already seen how anaemia is common in patients with chronic heart failure (CHF) and how it impacts on symptoms and prognosis. Increasingly, anaemia is being thought of as a possible target for treatment, and so an understanding of the possible causes of anaemia in CHF is vital for developing appropriate therapy (figure 1). Unfortunately, apart from unusual patients with specific pathologies underlying their anaemia, the origin of anaemia is multifactorial in most patients (table 1).
Haematinic deficiency
The commonest single haematinic deficiency related to anaemia in patients with CHF is iron deficiency. Around half of all patients with anaemia have evidence of iron deficiency on the basis of abnormal results for serum iron, iron binding capacity and ferritin.1,2 Folate or vitamin B12 deficiency is relatively uncommon. Iron is used by the body not only for haemoglobin production but in a variety of enzyme systems, which may be affected by iron deficiency.
There are many possible reasons for iron deficiency in patients with CHF. Dietary intake may be poor in the elderly population, and blood loss, too, is common. Although it is difficult to be prescriptive, the finding of iron deficiency should prompt gastroenterological investigations for possible underlying pathology. Gastrointestinal malignancy may be seen in patients with CHF as both conditions become more common with increasing age. If a patient has multiple haematinic deficiencies, such as iron and folate together, it should prompt a search for possible malabsorption, particularly coeliac disease.
The behaviour of erythropoietin (epo) in CHF is not completely clear. In some studies, epo has been found to be raised in proportion to the severity of heart failure, with increasing epo related to adverse outcome.3 Other investigators have found that the level of epo is lower than might be expected for the level of haemoglobin in some anaemic patients with CHF.4 The disparity might be related to the different assays used: some forms of epo are inactive, yet still measured in some assays.
Renal impairment
Renal impairment is extraordinarily common in patients with CHF, with more than half having a glomerular filtration rate less than 60 ml/min. Anaemia and iron deficiency are both more common in those with renal impairment.2 The outlook is worse for patients with both anaemia and renal dysfunction than with either alone.
The effects of CHF treatment
An irony of CHF treatment is that some of the successful treatments which improve prognosis are associated with anaemia. Angiotensin-converting enzyme (ACE) inhibitors, in particular, cause anaemia.5 The reason is not clear. A tetrapeptide, N-acetyl-seryl-aspartyl-lysyl-proline, is an endogenous inhibitor of erythropoiesis. It is broken down by ACE, so ACE inhibitors may prolong its effects, causing anaemia.6 In addition, angiotensin II tends to increase renal epo production via a reduction in renal blood flow: inhibiting angiotensin II therefore may contribute to a reduction in epo production.
It is worth noting that in COMET (the Carvedilol and Metoprolol European Trial), the more effective of the two beta blockers tested (carvedilol) was associated with a lower haemoglobin,7 suggesting that there may be some consequences of successful heart failure treatment in general that induce anaemia.
Aspirin can, of course, contribute to anaemia by its effects on blood loss and potentially via its renal effects. There is no good evidence that aspirin is beneficial in patients with CHF and so stopping it may be the correct approach in the patient with CHF and anaemia.
Haemodilution
It is difficult to measure fluid status precisely although it is undoubtedly the case that some patients with CHF are anaemic not because their red cell mass is low, but because their plasma volume is high and the red cells are diluted, giving the factitious impression of anaemia. In one study, two thirds of patients who were apparently clinically euvolaemic were in fact hypervolaemic.8
In a very careful study of 37 patients with heart failure and anaemia, around a half had dilutional anaemia and half had true anaemia – that is, a reduced red cell mass.9 However, this was a population of severely affected patients being assessed for possible heart transplantation, so it is difficult to know how generally applicable the results are.
The anaemia of chronic disease
Many chronic illnesses cause anaemia, and CHF is no exception. What all these conditions have in common is an association with systemic inflammation. The anaemia seems to be related to a primitive physiological response to inflammation (usually, in evolutionary terms at least, associated with infection): sequestering iron is a defence against invading bacteria, which are dependent upon its supply to multiply.
CHF is, of course, an inflammatory disease.10 Characteristic features of the anaemia of chronic disease are that iron is potentially available to the bone marrow, but is not being used. In association, epo may be reduced more than is expected for the haemoglobin level. Some studies have suggested that in patients with anaemia and CHF, up to 60% may have the anaemia of chronic disease, as suggested by low serum iron and iron-binding capacity but normal (or increased) ferritin.11
There has been much interest in hepcidin, a molecule produced by the liver in inflammatory states. Hepcidin reduces intestinal iron absorption and mobilisation from reticulo-endothelial stores, and might thereby contribute to iron deficiency and anaemia. However, more recent investigations have suggested that hepcidin may not be as important as first thought.12
Conclusion
Anaemia is common in patients with CHF but its origin is complex and imperfectly understood. In any individual patient, there may be more than one cause of anaemia, and in each patient anaemia should be investigated before embarking on treatment. It is only by understanding the origin of anaemia that appropriate treatment can be given: using a single treatment in every patient may cause harm.
Key messages
- The origins of anaemia in heart failure are multifactorial
- Its pathways are complex and not well understood
- There is no single treatment that will suit all patients
- Treatment must be based on an understanding of the causes of anaemia in each patient
References
- Witte KK, Desilva R, Chattopadhyay S, Ghosh J, Cleland JG, Clark AL. Are hematinic deficiencies the cause of anemia in chronic heart failure? Am Heart J 2004;147:924–30.
- de Silva R, Rigby AS, Witte KK et al. Anemia, renal dysfunction, and their interaction in patients with chronic heart failure. Am J Cardiol 2006;98:391–8.
- George J, Patal S, Wexler D et al. Circulating erythropoietin levels and prognosis in patients with congestive heart failure: comparison with neurohormonal and inflammatory markers. Arch Intern Med 2005;165:1304–09.
- Opasich C, Cazzola M, Scelsi L et al. Blunted erythropoietin production and defective iron supply for erythropoiesis as major causes of anaemia in patients with chronic heart failure. Eur Heart J 2005;26:2232–7.
- Ishani A, Weinhandl E, Zhao Z et al. Angiotensin-converting enzyme inhibitor as a risk factor for the development of anemia, and the impact of incident anemia on mortality in patients with left ventricular dysfunction. J Am Coll Cardiol 2005;45:391–9.
- van der Meer P, Lipsic E, Westenbrink BD et al. Levels of hematopoiesis inhibitor N-acetyl-seryl-aspartyl-lysyl-proline partially explain the occurrence of anemia in heart failure. Circulation 2005;112:1743–7.
- Komajda M, Anker SD, Charlesworth A et al. The impact of new onset anaemia on morbidity and mortality in chronic heart failure: results from COMET. Eur Heart J 2006;27:1440–6.
- Androne AS, Hryniewicz K, Hudaihed A, Mancini D, Lamanca J, Katz SD. Relation of unrecognized hypervolemia in chronic heart failure to clinical status, hemodynamics, and patient outcomes. Am J Cardiol 2004;93:1254–9.
- Androne AS, Katz SD, Lund L et al. Hemodilution is common in patients with advanced heart failure. Circulation 2003;107:226–9.
- Windram JD, Loh PH, Rigby AS, Hanning I, Clark AL, Cleland JG. Relationship of high-sensitivity C-reactive protein to prognosis and other prognostic markers in outpatients with heart failure. Am Heart J 2007;153:1048–55.
- Opasich C, Cazzola M, Scelsi L et al. Blunted erythropoietin production and defective iron supply for erythropoiesis as major causes of anaemia in patients with chronic heart failure. Eur Heart J 2005;26:2232–7.
- Divakaran V, Mehta S, Yao D et al. Hepcidin in anemia of chronic heart failure. Am J Hematol 2011;86:107–09.
Round table discussion
It seems surprising that treatments associated with improved outcomes in CHF such as ACE inhibitors (as discussed) and beta blockers can exacerbate anaemia. Why beta blockers are associated with anaemia is less clear. Aspirin can also cause blood loss and make patients iron deficient as well as exacerbating renal dysfunction. All agreed that the benefits of ACE inhibitors and beta blockers greatly outweigh this minor impact on haemoglobin.
Testing for iron deficiency
What is the best test to determine iron deficiency in CHF? In healthy individuals, ferritin levels are directly correlated with the total amount of body iron stored. Reduced levels suggest iron deficiency but different cut-off points are used to prompt intervention in patients with gastroenterological or gynaecological pathology compared to those with CHF. Because ferritin is an acute phase protein, its level may be affected by any inflammation. A transferrin saturation (TSAT) [reference range 15–50% in men; 12–45% in women] provides a measure of how much serum iron is actually bound and thereby available for use. A number of other variables may suggest iron deficiency, including low mean corpuscular volume (MCV), % hypochromic red cells, increased total iron binding capacity [TIBC] and increased soluble serum transferrin receptors. Not all of these tests are available in every haematology laboratory.
