There is conclusive evidence that lowering low-density lipoprotein (LDL) cholesterol levels with statins reduces the risk of cardiovascular disease events. However, it is also clear that a substantial residual cardiovascular risk persists, despite best treatment efforts.1,2 Some of this residual risk will be determined by modifiable risk factors, such as lipids, hypertension, tobacco use and diabetes. Further reducing apolipoprotein (apo) B-containing atherogenic lipoproteins or increasing atheroprotective lipoproteins, specifically raising high-density lipoprotein (HDL) cholesterol,2 are alternative proposed approaches to reducing this risk.
Other ways to reduce apoB lipoproteins
Based on regression analysis of major studies, further reduction of LDL cholesterol levels below current targets ( <1.5 mmol/L or 57 mg/dL) may have potential.3 Due to safety concerns with high-dose statin therapy, other approaches for reducing apoB levels are being investigated (table 1), although clinical outcomes data are so far not available.
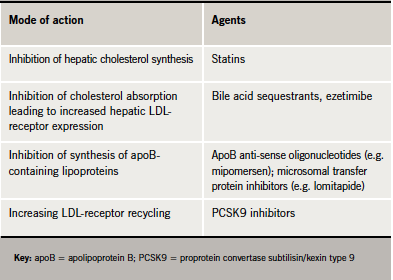
Reducing apoB synthesis (e.g. mipomersen, a novel anti-sense oligonucleotide) may be one approach. In moderate hyperlipidaemia (mean LDL cholesterol 4.47 mmol/L) treatment with mipomersen (200–300 mg/week) was associated with ∼45–60% reduction in LDL cholesterol and apoB-containing lipoproteins, and ∼50% reduction in triglycerides, exceeding that observed with statins.4 Mipomersen also reduced lipoprotein(a) (Lp[a]),4 recognised as an additional cardiovascular risk factor.5 However, the frequency of liver enzyme elevations and
risk of hepatic steatosis (fatty liver) require further evaluation.4
The nuclear receptors known as peroxisome proliferator-activated receptors (PPARs) and liver X receptors (LXRs) are lipid-activated transcription factors that have emerged as key regulators of lipid metabolism and inflammation. Agents acting at these receptors are under investigation. Additionally, eprotirome (KB2115), a thyroid hormone analogue that is poorly taken up by heart and bone, can favourably modulate lipid profiles. In statin-treated patients, eprotirome produced dose-related reductions in LDL cholesterol levels (by up to ∼30%). Similar reductions were also observed for triglycerides and Lp(a).6
Targeting LDL-receptor expression is another possibility. LDL is taken up through LDL-receptors on the cell surface. These LDL-receptors are either recycled back to the cell surface or are directed by proprotein convertase subtilisin/kexin type 9 (PCSK9) to degradation. A genetically upregulated PCSK9 is a cause of familial hypercholesterolaemia, while low PCSK9 activity protects against atheroma.7,8 These data suggest that inhibition of PCSK9 may have therapeutic potential.
Inhibition of microsomal transfer proteins (MTP), which are involved in the assembly of hepatic very-low-density lipoprotein (VLDL) and gut chylomicron lipoprotein particles, may be another target. The oral MTP inhibitor lomitapide, given as monotherapy or in combination with ezetimibe, lowered LDL cholesterol and apoB levels by ∼50% in patients with familial hypercholesterolaemia (FH) or moderate hypercholesterolaemia.9,10 However, the clinical utility of these agents may be restricted by their potential to induce elevation of liver enzymes and hepatic steatosis,9,10 although lomitapide was granted orphan drug designation in December 2010 for familial hyperchylomicronaemia.11 To overcome these adverse hepatic effects, non-absorbable, enterocyte-selective inhibitors of MTP are under investigation.12
Targeting non-LDL lipids
An alternative approach is to target non-LDL lipids. In post-hoc analyses of prospective trials in high-risk patients, elevated triglycerides or low plasma concentrations of HDL cholesterol have been associated with residual cardiovascular risk, even among patients achieving low LDL cholesterol.13,14 The evidence for elevated triglycerides alone as a cardiovascular risk factor is contentious. In contrast, observational studies conclusively established HDL cholesterol as an important predictor of cardiovascular risk, independent of LDL cholesterol levels and other lipid and non-lipid factors.15 The combination of low HDL cholesterol and elevated triglycerides, one of the defining features of the metabolic syndrome, is associated with increased cardiovascular risk.16
Targeting HDL cholesterol to reduce residual cardiovascular risk is, therefore, an attractive hypothesis. Nicotinic acid (niacin) is currently the only agent available that effectively raises HDL cholesterol, as well as lowering LDL cholesterol, triglycerides and Lp(a). In the Coronary Drug Project, treatment with nicotinic acid (immediate-release formulation 3 g/day) was associated with modest reduction in non-fatal myocardial infarction at the end of treatment and 11% reduction in all-cause mortality (p=0.0004) at 15 years (∼9 years after the end of treatment).17
However, the AIM-HIGH (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL Cholesterol/High Triglyceride and Impact on Global Health Outcomes) study18 was prematurely terminated 18 months ahead of schedule because extended-release nicotinic acid offered no additional benefits in these statin-treated patients with cardiovascular disease. A number of caveats need to be borne in mind; this event-driven trial was not sufficiently powered, achieved only a modest difference in HDL cholesterol and may have been compromised by the placebo containing a low dose of nicotinic acid (50 mg) to mask the study treatment. We need to wait for the results of the HPS2-THRIVE study in 25,000 participants, which is sufficiently powered to test the value of adding nicotinic acid to statin therapy.19
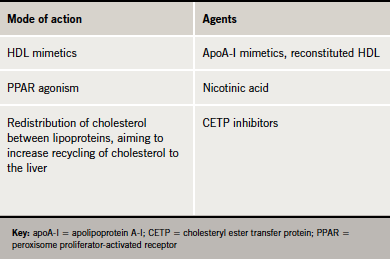
Several emerging HDL therapies, including reconstituted HDL, apoA-I mimetics and cholesteryl ester transfer protein (CETP) inhibitors may offer potential for increasing HDL cholesterol (table 2).
CETP in human plasma promotes transfer of cholesterol from HDL to LDL and triglyceride-rich lipoproteins such as VLDL and chylomicrons. Therefore, inhibition of CETP has the potential to shift the balance of plasma cholesterol in favour of the protective HDL fraction.20 Phase II trials with the CETP inhibitors currently in development have shown promising results.21-23 However, we need to wait for the results of ongoing major outcomes studies to evaluate whether these lipid changes translate to reduction in cardiovascular events. Additionally, we do not know whether HDL particle composition changes (and how achieved) will translate into functional change, for high HDL cholesterol per se might not augment cardiovascular protection.
Improving best practice: the way forward
These are exciting times in the development of new approaches aimed at targeting residual cardiovascular risk in statin-treated patients, and the results of clinical outcomes studies are awaited with interest.
For current practice, lifestyle intervention, statins and targeting low levels of HDL cholesterol and elevated triglycerides, which are characteristic of the metabolic syndrome and type 2 diabetes, are key issues. Better care, including counselling about therapeutic lifestyle intervention, and improving patient adherence are also priorities to improve practice and clinical outcomes.
Key messages
- Despite best treatment efforts, statin-treated patients often remain at a high risk of residual cardiovascular events
- Therapeutic interventions that further reduce apo B-containing atherogenic lipoproteins or raise atheroprotective lipoproteins, as in HDL may offer potential
- Results from ongoing outcomes studies are needed to evaluate the potential of nicotinic acid and the CETP inhibitors
- For current practice, clinicians should not overlook the value of therapeutic lifestyle intervention, as well as improving adherence with available pharmacological options
References
- Baigent C, Keech A, Kearney PM et al. Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90 056 participants in 14 randomised trials of statins. Lancet 2005;366:1267–78. http://dx.doi.org/10.1016/S0140-6736(05)67394-1
- Chapman J. Beyond LDL-cholesterol reduction: the way ahead in managing dyslipidaemia. Eur Heart J 2005;7(suppl F):F56–F62. http://dx.doi.org/10.1093/eurheartj/sui044
- O’Keefe JH Jr, Cordain L, Harris WH, Moe RM, Vogel R. Optimal low-density lipoprotein is 50 to 70 mg/dl: lower is better and physiologically normal. J Am Coll Cardiol 2004;43:2142–6. http://dx.doi.org/10.1093/eurheartj/ehr148
- Akdim F, Tribble DL, Flaim JD et al. Efficacy of apolipoprotein B synthesis inhibition in subjects with mild-to-moderate hyperlipidemia. Eur Heart J 2011;32:2650–9. http://dx.doi.org/10.1093/eurheartj/ehr148
- Nordestgaard BG, Chapman MJ, Ray K et al. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J 2010;31:2844–53. http://dx.doi.org/10.1093/eurheartj/ehq386
- Ladenson PW, Kristensen JD, Ridgway EC et al. Use of the thyroid hormone analogue eprotirome in statin-treated dyslipidemia. N Engl J Med 2010;362:906–16. http://dx.doi.org/10.1056/NEJMoa0905633
- Humphries SE, Neely RD, Whittall RA et al. Healthy individuals carrying the PCSK9 p.R46L variant and familial hypercholesterolemia patients carrying PCSK9 p.D374Y exhibit lower plasma concentrations of PCSK9. Clin Chem 2009;55:2153–61. http://dx.doi.org/10.1373/clinchem.2009.129759
- Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 2006;354:1264–72.
- Cuchel M, Bloedon LT, Szapary PO et al. Inhibition of microsomal triglyceride transfer protein in familial hypercholesterolemia. N Engl J Med 2007;356:148–56. http://dx.doi.org/10.1056/NEJMoa061189
- Samaha FF, McKenney J, Bloedon LT, Sasiela WJ, Rader DJ. Inhibition of microsomal triglyceride transfer protein alone or with ezetimibe in patients with moderate hypercholesterolemia. Nat Clin Pract Cardiovasc Med 2008;5:497–505. http://dx.doi.org/10.1038/ncpcardio1250
- Lomitapide EU orphan designation number EU/3/10/823. Available from: http://ec.europa.eu/health/documents/community-register/html/o823.htm [accessed 4 January 2012].
- Lilly SM, Rader DJ. New targets and emerging therapies for reducing LDL cholesterol. Curr Opin Lipidol 2007;18:650–5. http://dx.doi.org/10.1097/MOL.0b013e3282f169c6
- Miller M, Cannon CP, Murphy SA et al.; PROVE IT-TIMI 22 Investigators. Impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE IT-TIMI 22 trial. J Am Coll Cardiol 2008;51:724–30. http://dx.doi.org/10.1016/j.jacc.2007.10.038
- Barter P, Gotto AM, LaRosa JC et al.; Treating to New Targets Investigators. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med 2007;357:1301–10. http://dx.doi.org/10.1056/NEJMoa064278
- The Emerging Risk Factors Collaboration. Major lipids, apolipoproteins, and risk of vascular disease. JAMA 2009;302:1993–2000. http://dx.doi.org/10.1001/jama.2009.1619
- Chapman MJ, Ginsberg HN, Amarenco P et al.; European Atherosclerosis Society Consensus Panel. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur Heart J 2011;32:1345–61. http://dx.doi.org/10.1093/eurheartj/ehr112
- Canner PL, Berge KG, Wenger NK et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol 1986;8:1245–55. http://dx.doi.org/10.1016/S0735-1097(86)80293-5
- The AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011;365:2255–67. http://dx.doi.org/10.1056/NEJMoa1107579
- HPS2-THRIVE study. Available from: http://www.hps2-thrive.org/ [accessed 24 January 2012].
- Barter PJ, Brewer HB Jr, Chapman MJ, Hennekens CH, Rader DJ, Tall AR. Cholesteryl ester transfer protein: a novel target for raising HDL and inhibiting atherosclerosis. Arterioscler Thromb Vasc Biol 2003;23:160–7. http://dx.doi.org/10.1161/01.ATV.0000054658.91146.64
- Stein EA, Roth EM, Rhyne JM et al. Safety and tolerability of dalcetrapib (RO4607381/JTT-705): results from a 48-week trial. Eur Heart J 2010;31:480–8. http://dx.doi.org/10.1093/eurheartj/ehp601
- Cannon CP, Shah S, Dansky HM et al. The DEFINE Investigators. Safety of anacetrapib in patients with or at high risk for coronary heart disease. N Engl J Med 2010;363:2406–15. http://dx.doi.org/10.1056/NEJMoa1009744
- Nicholls SJ, Brewer B, Kastelein JJP et al. Effects of the CETP inhibitor evacetrapib administered as monotherapy or in combination with statins on HDL and LDL cholesterol. JAMA 2011;306:2099–109. http://dx.doi.org/10.1001/jama.2011.1649