Although morbidity and mortality are the most commonly used end points in clinical trials of heart failure treatments, it is also important to consider how patients experience their illness when assessing treatment efficacy. The goal of this study was to use qualitative interviews to identify key experiences that may be targeted as end points in future heart failure trials.
Interviews were conducted with 63 chronic heart failure patients. Interview responses were coded using ATLAS.ti software. Code frequency and bother ratings were used to identify salient patient experiences. Key symptoms included shortness of breath, tiredness, swelling of the lower extremities, and pain (chest and other). Shortness of breath and tiredness were often described as being related to physical activities. Key areas impacted by heart failure included physical activity and mobility limitations, and a variety of emotional effects.
In conclusion, patients report a number of symptoms and impacts related to heart failure. Although some experiences are already widely captured in clinical and patient-reported heart failure assessments, others, such as pain, are not. These findings support the use of patient-reported outcome instruments as end points when assessing the efficacy of heart failure treatments.
Introduction
Clinical trials for new heart failure treatments have traditionally focused on mortality and hospitalisations as primary end points.1,2 Although clearly important, these end points tell us little about how heart failure (HF) patients experience their illness and treatment in their day-to-day life. HF may affect patients’ quality of life more than many other chronic diseases, including diabetes and arthritis.3,4 Symptoms and quality of life, as reported by patients, are correlated with mortality and hospitalisations,5-7 suggesting that these concepts may be indicative of an underlying process that ultimately manifests in death and/or increased healthcare resource utilisation. For these reasons, it appears that it would be useful to better understand what patients experience outside of the clinic; this may ultimately improve how HF treatments are evaluated.
Patient-reported outcome measures (PROMs or PROs) provide a mechanism for assessing the patient’s day-to-day experience, and can be important in evaluating the efficacy of HF treatments. PROMs are defined as “any report of the status of a patient’s health condition that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else”.8 PROMs have been gaining increasing attention in the UK as markers of treatment benefit. The National Institute for Health and Clinical Excellence (NICE) overview of methods used in health technology assessment highlights the value of the patient perspective and health-related quality of life.9 Additionally, since 2009, the UK Department of Health has required providers of certain surgical procedures to collect PROMs data before and after the procedure.10 This type of initiative may lead to more frequent use of PROMs at a national and local level, in order to understand patient perspectives on clinical care.11 More broadly, both the European Medicines Agency (EMA) and Food and Drug Administration (FDA) in the USA have issued guidelines that highlight the utility of PROMs in clinical development programmes.8,12 In order to continue to refine PROMs measurement strategies with HF patients, we must first understand what aspects of the patients’ experience are important to measure. Accordingly, the primary goal of this study was to identify key experiences that may be assessed by PROMs in future HF trials.
The goal of this study was to identify concepts that are relevant to chronic HF patients via a qualitative patient interview process. It answers the simple, but critically important question: what do chronic HF patients experience? This information can be combined with expert clinical insights, in order to guide the creation of new PROMs in future clinical trials with HF patients. The data can also be used to examine the content validity of existing HF PROMs that might be used in clinical trials or clinical practice.
Materials and methods
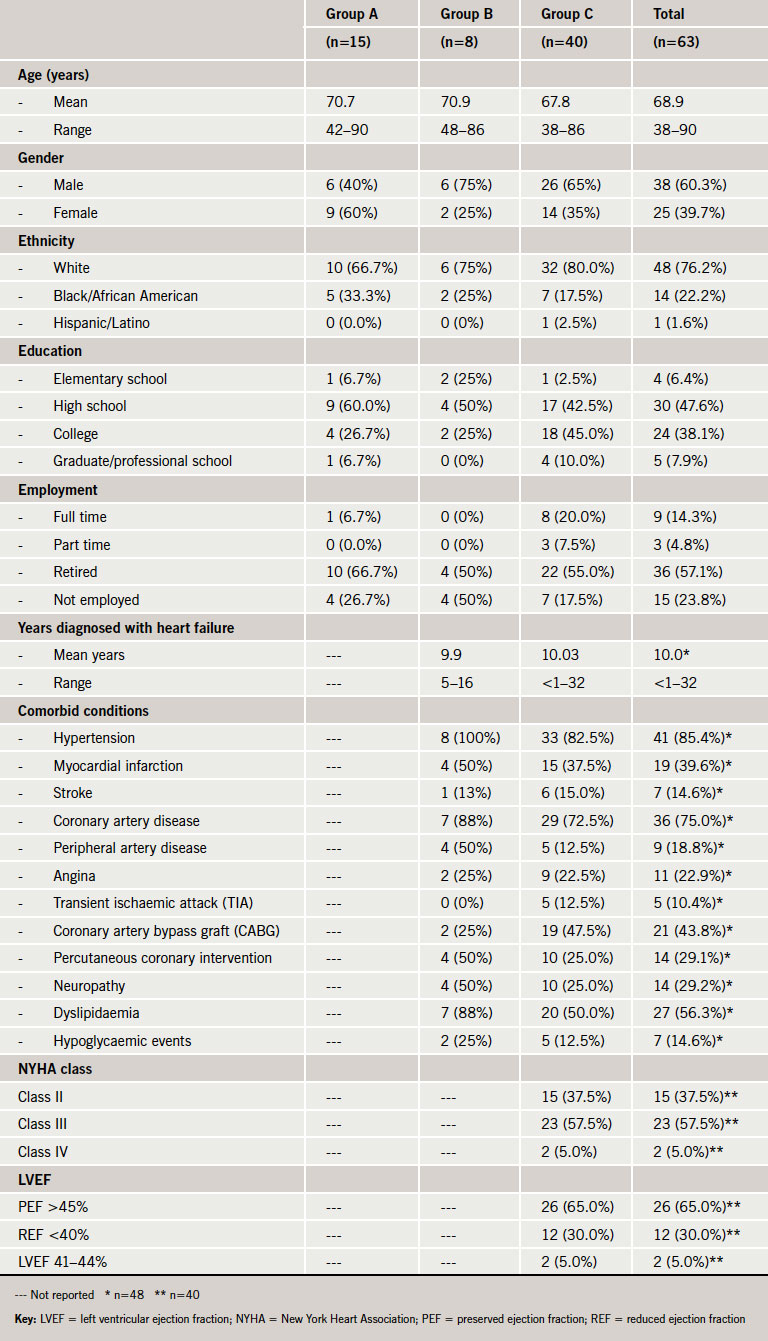
Individual patient interviews were conducted with 63 patients with chronic HF in six locations across the USA. The 63 patients are from three separate studies (groups A, B and C below). Although results for these studies are presented separately, the similarity between patient groups and interview methods also allowed the presentation of pooled data. While specific aims for the three groups had slight differences, the overall goal was to identify relevant and important concepts to patients with chronic HF, and to understand broadly the language that they use to describe their experiences. The interviewers in the study had between five and 10 years of qualitative research experience, including two years of experience in cardiovascular disease. The interviewers were clinically trained: one as a naturopathic clinician and the other as a hospice and crisis line counsellor. Both interviewers were trained for this specific study and the interview guide used in the study was pilot tested prior to starting the study.
- Group A (n=15): goal was to elicit all relevant concepts, with a particular focus on the concepts found in the Kansas City Cardiomyopathy Questionnaire (KCCQ) in order to retrospectively evaluate its content validity (analysis of KCCQ content validity presented elsewhere13).
- Group B (n=8): goal was to elicit all relevant concepts.
- Group C (n=40): goal was to evaluate relevant concepts in patients with preserved ejection fraction (PEF) and reduced ejection fraction (REF) HF.
Patients were enrolled if they were at least 18 years of age, provided informed consent, had a physician-documented diagnosis of chronic HF, were New York Heart Association (NYHA) class II–IV, had been stable for three months or longer, and were able to read, write and speak English and understand the interview questions.
For all groups, patients were excluded if they had a major cardiac event within the past three months, had a clinical condition that would interfere with their participation in the interview, or were diagnosed with a condition that could affect their ability to distinguish the symptoms of HF. Participants were also excluded if they were known to be pregnant or were currently participating in another study. These inclusion and exclusion criteria served to aid in the selection of a sample of patients that are similar to clinical trial samples by virtue of their diagnoses and severity levels.
Study candidates were identified from clinic medical records. Medical records were reviewed to determine which patients met the inclusion and exclusion criteria for the study and those who did were contacted by phone to determine interest and willingness to participate.
The interviewers used a semi-structured interview guide to elicit both spontaneous and prompted descriptions of the patient’s experiences with HF. The interview began with open-ended questions that were designed to allow the patient to describe their experiences without prompting. After spontaneous reports were exhausted, patients were directly asked about specific symptoms and other experiences (such as emotional, social, or occupational effects) that may be related to HF or its treatment. These prompts were selected based on concepts identified from the published literature, existing measures, and discussions with clinical experts. Given the degree of comorbidity between HF and depression,14 patients were directly prompted about depressed mood, if it was not discussed in response to open-ended questioning. Fifty-five patients (groups A and C) were asked to rate the symptom and impact that is most bothersome or the most important to eliminate (‘to be rid of’). The patients received a small fee for their participation in the study.
Each interview session lasted approximately 60 minutes. Audiotapes from all interviews were transcribed and used for the coding and content analysis process. The transcripts were loaded into the ATLAS.ti (version 5.0) software program15 for content analysis. Relevant passages from the transcripts were assigned a descriptive code using a coding dictionary. The coding dictionary initially included concepts identified in expert interviews and a review of the published literature. Coders followed these steps:
- The coder identified each time in the transcript text where the patient expressed a concept.
- This text was tagged within the ATLAS.ti program.
- The coder then matched the tagged text (by content) to a code stem from the coding dictionary (or created a new code stem if needed).
This mixed deductive and inductive approach to developing concept codes was meant to ensure that the ideas generated from patients during the interview process would have appropriate influence on both the variety of the codes assigned and the overall organisation of the qualitative results. Similar codes were grouped into categories and these categories were used as the unit of analysis.
The data coding personnel were trained qualitative data analysts. Inter-rater agreement was evaluated in terms of identifying patient interview transcript text to be coded (step 1 above; 81.8–95.5% agreement) as well as the specific code assigned (step 3 above; 97.3–100% agreement). Concept saturation – the point at which no new information is obtained from new interviews – was used to evaluate the adequacy of the sample size.16
The codes assigned to patient expressions were tabulated in order to present their frequency of appearance in the patient interviews. When a concept is of particular significance to a patient, the patient may express that concept multiple times, with each ‘expression’ being recorded and tabulated separately. A summary table was produced showing the number of patients who expressed a concept and the number of times that each concept was expressed. The number of patients reporting a concept reflects the generalisability of the concept to the target patient population.17
Finally, symptom and impact bother rankings were summarised for each concept as a final step in evaluating the HF patients’ experiences. Evaluating all of these streams of information (number of concept expressions, number of patients expressing a concept, and degree of bother related to each concept) provides an understanding of the most salient concepts to chronic HF patients.
Results
Demographic and clinical characteristics of the sample are presented in table 1. The mean age was 68.9 years (ranging from 38 to 90 years of age). Participants were mostly male (60.3%) and white (76.2%). Participants had a variety of comorbid conditions, which is common in patients with HF. NYHA class was recorded for group C only, and most participants in that group were identified as being NYHA class III (57.5%); however, there was representation of patients with both class II (37.5%) and class IV (5.0%). Saturation was achieved in the interview sample indicating that the sample size was adequate – no new information is likely to be gained by conducting additional interviews with this target population.
HF symptom experience
For group C, results were first reviewed for PEF and REF patients separately; as no major differences were found between the groups, results are presented for the total group of HF patients.
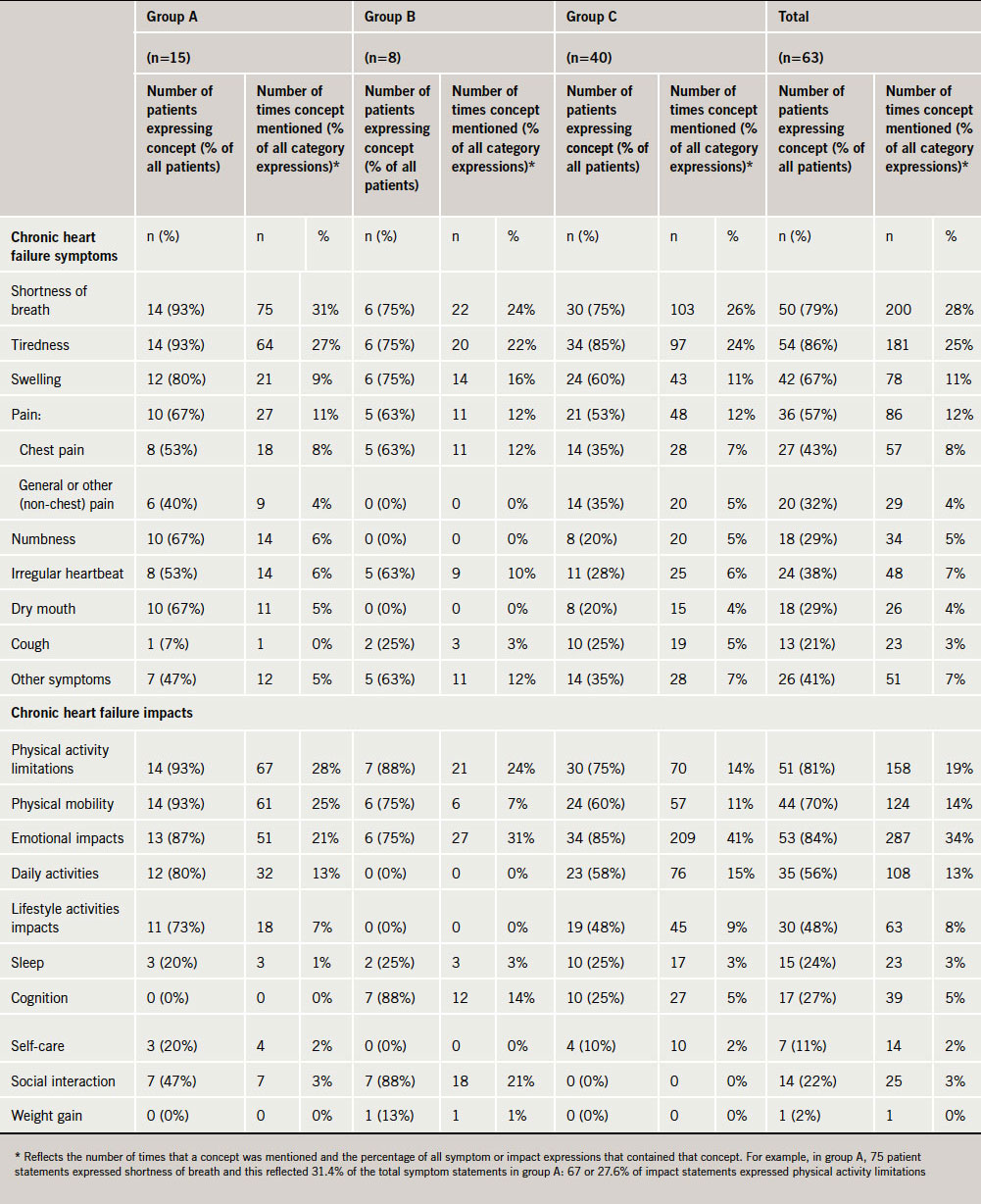
As shown in table 2, three tiers of symptoms consistently emerged in the interviews. The first tier includes shortness of breath and tiredness, which were the two most commonly mentioned symptoms. Not only did the vast majority of patients mention shortness of breath and tiredness, they also mentioned them multiple times within the interview, which highlights the importance of the concepts. The second tier included swelling (primarily of the lower extremities) and pain. The remaining symptoms fall into tier three, being mentioned far less frequently than those in tiers one or two.
Patients described becoming out of breath when performing activities such as walking up steps, walking around the house or to the car, and doing housework.
- “Just walking in here from the parking lot, I got very short of breath.”
- “It seems like I can start walking down the hall and literally have to lean up against the wall for a second because I am gasping for breath.”
A large number of patient expressions for the sub-domain of breathing problems at nighttime when lying down were also identified.
- “I couldn’t breathe. I woke up in the middle of the night and I was real short of breath.”
- “It gets so bad that even in the night when I sleep or if I am even turning over, I will have to gasp for breath.”
Similar to breathing problems, patients often described fatigue and tiredness in relation to performing activities such as housework, walking and shopping.
- “I can’t sweep, mop or run the vacuum cleaner. I get totally exhausted.”
- “I’m not able to do a lot of things that I would like to do because I’m tired a lot.”
Swelling in the legs, ankles and feet was the primary type of swelling reported. Chest pain was the most common type of pain described by patients, but they also reported experiencing pain in other areas, including back, leg and arm pain. However, these experiences were reported somewhat less frequently than chest pain.
- “I had quite a few chest pains during the day and everything, like if I had to walk up a flight of stairs I’d hurt and stuff.”
- “Originally [before diagnosis of a heart condition], I went to the doctor because I had pain in the middle of my back, a lot of pain.”
- “Every time I lay down to sleep at night, my hands will tingle like they are asleep, but then they hurt and burn. It is really painful.”
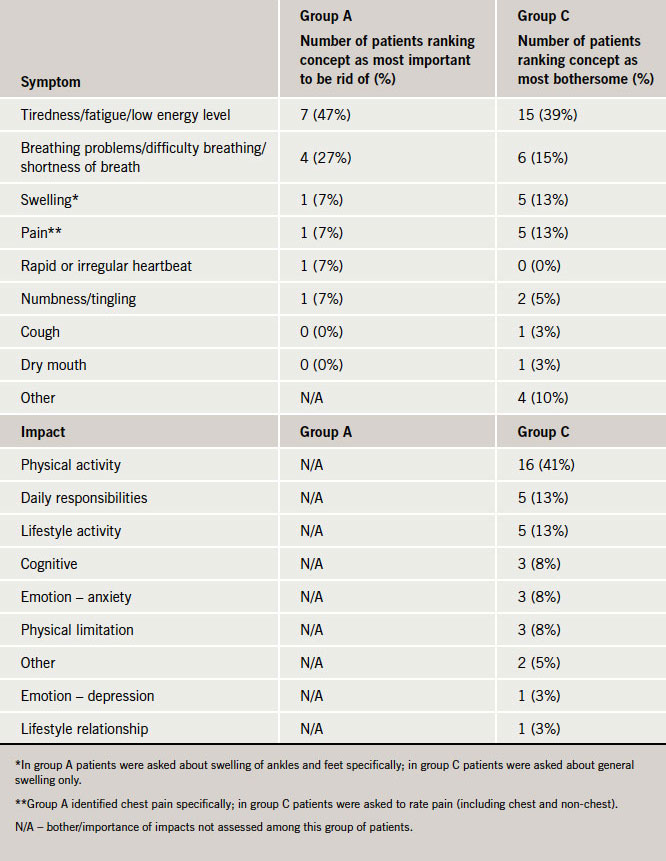
As shown in table 3, patients consistently ranked tiredness and shortness of breath as the most bothersome or the most important symptoms that they would like to eliminate, followed by swelling and pain.
Impact of illness and symptoms
The most frequently mentioned HF impacts related to physical limitations and mobility problems. For example, with the exception of one individual, all participants in group A (93%) reported physical limitations and physical mobility impacts as primary impacts of their HF. Difficulties with mobility most often included difficulty walking and carrying objects. Impacts on daily activities and responsibilities (such as housework and paid work) and lifestyle activities (such as hobbies and social activities) were also frequently identified and mentioned by patients in groups A and C (table 2). Emotional impacts were often mentioned by patients, and these impacts varied considerably including expressions of annoyance, anxiety, irritation, frustration, depression, embarrassment, guilt and fear. Only patients in group C were asked about how bothersome HF impacts were. Physical activity limitations were most frequently described as the most bothersome by patients (41%).
Discussion
The qualitative data from this study suggest that patients experience a variety of negative effects due to HF in their day-to-day lives. The key symptoms that emerged consistently across three patient interview groups included shortness of breath, tiredness, swelling (particularly of the legs, feet, ankles) and pain (chest and other). Key impacts included physical activity and mobility limitations. These findings are largely consistent with previous qualitative work among HF patients, which identifies shortness of breath and lack of energy as the most prevalent symptoms, and physical limitations as the most relevant impact area.18-20
The key symptoms identified by patients were characterised as occurring at rest, but were particularly noticeable during physical activity. This is important to consider when assessing symptom severity in clinical trials, as degree of engagement in physical activities may partially determine symptom severity. It may be useful to measure symptoms in relation to varying levels of physical activity. Specific activities to address may include mobility concerns (i.e. walking, lifting and carrying, and climbing stairs), as well as activities that range in intensity from mild to strenuous, and include, among others, housework, yard work, and exercising or playing sports.
Pain emerged as a potentially important concept, with patients reporting chest pain and non-chest pain. Patient-reported pain in HF has been described previously21 and has been identified in other qualitative studies of HF patients. For example, 38% of chronic HF patients presenting at the emergency department for worsening HF report experiencing pain, with approximately 30% reporting chest pain and 20% reporting other types of pain.22 The current study demonstrates that chronic HF patients in a non-acute setting continue to report notable pain. The relationship between pain and HF is not well understood, as it is not clear if pain is a key symptom of the condition, or if pain is a common comorbidity. Pain may result from HF-related swelling or impaired circulation to organs.23 Additionally, pain perception may be altered by common HF symptoms (e.g. shortness of breath and fatigue), as well as emotional impacts such as anxiety and depression.23 Existing PROMs in HF do not assess pain, reflecting the assumption that pain is not a key feature of the condition. It will be important to better understand if pain is a core feature of HF before making a determination about assessing pain to evaluate treatment effects. The fact that it is often mentioned by HF patients in this study warrants further attention.
Understanding and incorporating HF patients’ perspectives on their illness and treatment in clinical trials is an important goal for the healthcare industry. PROMs can help identify the burden of illness on patients, which will help clinicians and payers understand the value of treatments beyond their impact on physiological or hospitalisation end points. As noted earlier, key stakeholders in the UK have highlighted the importance of using PROMs to understand treatment benefit. The 2008 National Health Service (NHS) High Quality Care for All report emphasises the potential central role of PROMs when assessing effectiveness alongside clinical measures: “Just as important is the effectiveness of care from the patient’s own perspective which will be measured through patient-reported outcomes measures (PROMs).”24 The findings from the qualitative interviews in this study may have significant implications for PROMs used in HF clinical trials. The concepts identified as part of this study appear to represent areas of importance to patients and, therefore, should be considered for inclusion in any PROMs that are used to examine the efficacy of HF treatments.
One possible limitation of this research is that the sample size used in this study may seem small, particularly in comparison to clinical trials. However, this sample size is not unusual in qualitative research. Additionally, evidence of concept saturation was found in all three groups. This suggests that the sample size was adequate to support the broad objective of comprehensively identifying relevant and important concepts to HF patients. Although the formats of the three interview groups were similar enough to allow pooling of the data, it does increase complexity in interpreting the data. For example, NYHA class and left ventricular ejection fraction (LVEF) were only recorded for one of the three groups; severity of chronic HF experience is not documented for two groups. Finally, these results were obtained exclusively from US patients. It may be useful to conduct interviews with patients from other regions, in order to explore potential differences in symptom and impact experience.
The results from these interviews demonstrate that symptoms (overall and related to activities) including shortness of breath, tiredness, swelling and, potentially, pain, as well as physical limitations, are key to the patient’s experience of the disease burden in HF. PROMs including these concepts may be used as end points in clinical trials of treatments for HF, in order to better understand the day-to-day impact of disease and treatment efficacy •
Conflicts of interest
This study was funded by Novartis Pharmaceuticals Corporation. CJG: none declared. AFS: none declared. MM: receives research funds from Novartis to conduct data collection studies. RA: is an employee of and holds stock in Novartis Pharmaceuticals Corporation. YB: is an employee of and holds stock in Novartis Pharma AG.
Key messages
- Heart failure patients may experience a variety of symptoms and limitations in their daily lives that may be appropriate targets for treatment in clinical trials. Qualitative interviews were used in this trial to elicit relevant patient experiences
- Key symptoms include shortness of breath, tiredness, swelling of the lower extremities and pain (chest and general). Pain is not commonly recognised as a symptom of heart failure, but was reported frequently by patients in this study. It will be necessary to further examine the role of pain in heart failure
- Patients reported a number of impacts on their lives due to heart failure, including limitations in mobility and physical activities, and emotional distress. Limitations in physical activities, such as doing household chores, exercising and playing sports, were noted as particularly bothersome
- Without understanding the impact of heart failure interventions on patients’ everyday lives, it is difficult to have a complete understanding of treatment efficacy. Patient reported outcome measures may be used to assess these key patient experiences
References
- Krum H, Carson P, Farsang C et al. Effect of valsartan added to background ACE inhibitor therapy in patients with heart failure: results from Val-HeFT. Eur J Heart Fail 2004;6:937–45. http://dx.doi.org/10.1016/j.ejheart.2004.09.005
- Solomon SD, Wang D, Finn P et al. Effect of candesartan on cause-specific mortality in heart failure patients: the candesartan in heart failure assessment of reduction in mortality and morbidity (CHARM) program. Circulation 2004;110:2180–3. http://dx.doi.org/10.1161/01.CIR.0000144474.65922.AA
- Stewart AL, Greenfield S, Hays RD et al. Functional status and well being of patients with chronic conditions. Results from the Medical Outcomes Study. JAMA 1989;262:907–13. http://dx.doi.org/10.1001/jama.1989.03430070055030
- Hobbs FDR, Kenkre JE, Roalfe AK, Davis RC, Hare R, Davies MK. Impact of heart failure and left ventricular systolic dysfunction on quality of life: a cross sectional study comparing common chronic cardiac and medical disorders and a representative adult population. Eur Heart J 2002;23:1867–76. http://dx.doi.org/10.1053/euhj.2002.3255
- Ekman I, Cleland CJF, Swedberg K, Charlesworth A, Metra A, Poole-Wilson PA. Symptoms in patients with heart failure are prognostic predictors. Insights from COMET. J Card Fail 2005;11:288–92. http://dx.doi.org/10.1016/j.cardfail.2005.03.007
- Ekman I, Kjork E, Andersson B. Self-assessment symptoms in chronic heart failure: important information for clinical management. Eur J Heart Fail 2007;9:424–8. http://dx.doi.org/10.1016/j.ejheart.2006.10.020
- Alla F, Briancon S, Guillemin F et al. Self-rating of quality of life provides additional prognostic information in heart failure. Insights into the EPICAL study. Eur J Heart Fail 2002;4:337–43. http://dx.doi.org/10.1016/S1388-9842(02)00006-5
- Food and Drug Administration. Guidance for industry. Patient reported outcome measures: use in medical product development to support labeling claims. FDA [serial online] 2009; accessed March 14, 2010.
- National Institute for Health and Clinical Excellence. Guide to methods of technology appraisal. London: NICE, 2008.
- NHS Department of Health. Guidance on the routine collection of patient reported outcomes measures (PROMs). London: DoH, 2009.
- Dawson J, Doll H, Fitzpatrick R, Jenkinson C, Carr AJ. The routine use of patient reported outcome measures in healthcare settings. BMJ 2010;340:c186. http://dx.doi.org/10.1136/bmj.c186
- European Medicines Agency. Reflection paper on the regulatory guidance for the use of health related quality of life (HRQL) measures in the evaluation of medicinal products. London: EMA, 2005.
- Heart failure patient insights reflected in the Kansas City Cardiomyopathy Questionnaire: poster presentation. American Heart Association Quality of Care and Outcomes Research in Cardiovascular Disease and Stroke Scientific Sessions; 2010.
- Royal College of Physicians. Chronic heart failure: national clinical guideline for diagnosis and management in primary and secondary care. London: National Clinical Guideline Centre at The Royal College of Physicians, 2010.
- Muhr T. User’s manual for ATLAS.ti 5.0. Berlin: ATLAS.ti Scientific Software Development GmbH, 2004.
- Guest G, Bunce A, Johnson L. How many interviews are enough: an experiment with data saturation and variability. Field Methods 2006;18:59–82. http://dx.doi.org/10.1177/1525822X05279903
- ISPOR good research practices for establishing and reporting evidence of the content validity of new PRO instruments. Panel discussion at the annual meeting of the International Society for Pharmacoeconomics and Outcomes Research. International Society for Pharmacoeconomics and Outcomes Research (ISPOR); 10
May, 2010. - Zambroski CH, Moser DK, Bhat G, Ziegler C. Impact of symptom prevalence and symptom burden on quality of life in patients with heart failure. Eur J Cardiovasc Nurs 2005;4:198–206. http://dx.doi.org/10.1016/j.ejcnurse.2005.03.010
- Dunderdale K, Furze G, Thompson DR, Beer SF, Miles JNV. Health related quality of life from the perspective of patients with chronic heart failure. Br J Cardiol 2007;14:207–12.
- Bosworth HB, Steinhauser KE, Orr M. Congestive heart failure patients’ perception of quality of life: the integration of physical and psychosocial factors. Aging Ment Health 2004;8:83–91. http://dx.doi.org/10.1080/13607860310001613374
- Goebel JR, Doering LV, Evangelista LS et al. A comparative study of pain in heart failure and non-heart failure veterans. J Card Fail 2009;15:24–30. http://dx.doi.org/10.1016/j.cardfail.2008.09.002
- Patel H, Shafazand M, Schaufelberger M, Ekman I. Reasons for seeking acute care in chronic heart failure. Eur J Heart Fail 2007;9:702–08. http://dx.doi.org/10.1016/j.ejheart.2006.11.002
- Goodlin SJ, Wingate S, Pressler SJ, Teerlink JR, Storey CP. Investigating pain in heart failure patients: rationale and design of the pain assessment, incidence and nature in heart failure (PAIN-HF) study. J Card Fail 2008;14:276–82. http://dx.doi.org/10.1016/j.cardfail.2008.01.008
- Darzi A. High quality care for all: NHS next stage review final report. London: Department of Health,
June 2008.
