Abnormalities in plasma lipoprotein concentrations are found in seven of out every 10 patients with premature coronary disease, with a familial disorder in more than half of these cases, highlighting the importance of accurate diagnosis and scope for early treatment of affected families.1 Clinical assessment, incorporating review of phenotypic features, personal and family history, physical signs and laboratory tests, is fundamental to diagnosis.
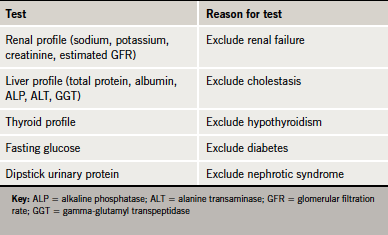
In the first instance, it is important to exclude secondary causes of dyslipidaemia. Diabetes mellitus, untreated hypothyroidism, nephrotic syndrome, obesity and cholestasis are all associated with hyperlipidaemia and must be excluded by baseline biochemical tests (table 1 and see also the case scenario on page S11).
In addition, a wide range of medications are commonly implicated as a cause of dyslipidaemia, including:
- atypical antipsychotics, corticosteroids and ciclosporin, which increase cholesterol and triglycerides
- beta blockers, HIV/antiretroviral drugs, oestrogens and retinoids, which increase triglycerides
- anabolic steroids, which lower high-density lipoprotein (HDL) cholesterol.
For many people with primary dyslipidaemia, cholesterol is relatively easy to manage once diagnosed. However, clinicians must consider the possibility of a familial aetiology, especially in those patients with a strong family history of coronary heart disease (CHD).2 Such disorders are commonly underdiagnosed in practice. With early diagnosis and instigation of appropriate lipid-lowering therapy and therapeutic lifestyle changes, major cardiovascular complications can be prevented.
Familial hypercholesterolaemia
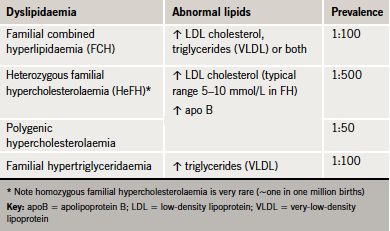
In the absence of secondary causes, a strong family history of premature CHD is suggestive of an atherogenic familial lipid disorder (table 2). Of these, familial hypercholesterolaemia (FH) is the best recognised, with an estimated prevalence in Caucasians of one in 500 (0.2%).3,4 This means that in the UK, about 110,000 people have FH.4 Almost all affected people are heterozygotes (HeFH). Homozygous FH is extremely rare (~one in one million).2
Low-density lipoprotein (LDL) cholesterol levels in individuals with HeFH are typically double normal levels, in the range of 5–10 mmol/L.2 HeFH increases the risk of premature CHD dramatically, with a cumulative risk of 50% in men by age 50 and 30% in women by age 60.4 If affected individuals are not diagnosed and treated, 50% of men and 15% of women will have died by age 60. However, if individuals are diagnosed and treated they can look forward to a normal life expectancy.2
FH is due to an autosomal genetic defect affecting the LDL-receptor pathway.5 Normally LDL-derived cholesterol acts at several levels, to suppress transcription of the LDL-receptor gene. This allows cells to regulate the number of LDL-receptors to provide sufficient cholesterol for metabolic needs. When cholesterol levels in the cell increase, the production of LDL-receptors is decreased. FH may be caused by mutations in genes coding for the LDL-receptor (LDLR), apolipoprotein (apo) B100 (the LDL-receptor ligand), and a protease known as PCSK9 (proprotein convertase subtilisin/kexin type 9), which is involved in the regulation of LDL-receptor recycling.5
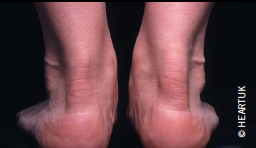
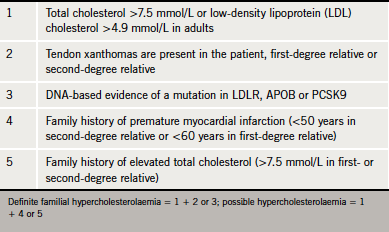
The finding of tendon xanthomas confirms the diagnosis of FH. Although these are found in fewer than 30% of cases, greater than 80% of such cases will have a disease defining mutation in LDLR, APOB or PCSK9 genes (figure 1).4 Studies have also shown that the presence of this clinical sign is associated with a significant increase in cardiovascular disease risk across all age groups.6 The National Institute for Health and Clinical Excellence (NICE) guidelines recommend cascade screening involving a combination of genetic testing and LDL cholesterol measurement for definitive diagnosis (table 3). If a familial FH mutation is not identified or genetic testing is not available, relatives of an FH patient can be diagnosed on the basis of sex- and age-specific LDL cholesterol thresholds (as recommended by NICE).4 Diagnosis on clinical signs alone is less sensitive.
Underdiagnosis remains a major problem, with estimates suggesting that less than 25% of people with FH are diagnosed.7 As FH is readily treatable with statins,4 there is clearly a role for primary care in the diagnosis and identification of affected family members with FH. A nationwide, proactive, systematic approach to cascade testing is recommended in guidelines,2,4 but commissioning support for implementation is lacking in most parts of the UK.
Combined hyperlipidaemia
A lipid profile defined by elevated LDL cholesterol, triglycerides or both can be suggestive of a number of dyslipidaemias. These include familial combined hyperlipidaemia (FCH), remnant hyperlipidaemia (Type III or familial dysbetalipoproteinaemia) and dyslipidaemia associated with the metabolic syndrome, as well as milder presentations of familial hypertriglyceridaemia (see the case scenario on page S8).
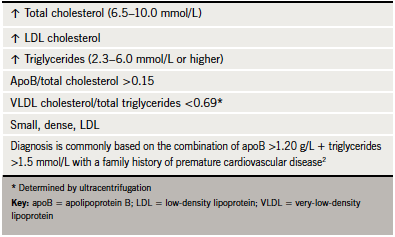
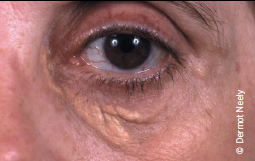
FCH affects about one in 100 people. The underlying mechanism involves overproduction of very-low-density lipoprotein (VLDL) and apoB. The genetic basis is complex and multifactorial and also influenced by environmental factors.2 As there is considerable variability in presentation, diagnosis can often be missed in practice. FCH should be suspected if total cholesterol levels are in the range 6.5–8.0 mmol/L and/or triglycerides between 2.3 and 5.0 mmol/L (table 4). Elevated levels, either alone or in combination, in patients and other family members confer a ‘variable phenotype’. ApoB is invariably elevated and is, therefore, a useful diagnostic tool, with levels >1.20 g/L, together with elevated triglycerides and family history of cardiovascular disease, strongly suggestive of diagnosis.2 The finding of an apoB concentration that is unexpectedly low (apoB/total cholesterol ratio <0.15 g/mmol) raises suspicion of remnant hyperlipidaemia.8 The presence of xanthelasma (figure 2) is not of diagnostic significance but represents an area of lipid-laden macrophages, which is predictive of an increased risk of CHD, atherosclerosis and mortality.9
Lipid profiles
Clearly the full lipid profile is key to diagnosis of dyslipidaemia. The baseline lipid evaluation should comprise total cholesterol, triglycerides and HDL cholesterol.2 Because total cholesterol involves measurement of both atherogenic (LDL-, intermediate-density lipoprotein [IDL]- and VLDL cholesterol) and anti-atherogenic (HDL cholesterol) lipid fractions, it is inadequate for monitoring treatment. Instead, LDL- and non-HDL cholesterol are preferred. Of the two, there is a strong case for preferential use of non-HDL cholesterol, given that 1) it is a simple calculation (non-HDL cholesterol = total cholesterol – HDL cholesterol); and 2) can be readily measured in non-fasting samples.10 In contrast, LDL cholesterol must be measured in fasting samples as its calculation assumes a constant cholesterol/triglyceride ratio in VLDL of 0.45. This assumption does not hold in non-fasting conditions or when the fasting triglyceride concentration exceeds 4.5 mmol/L.2 Furthermore, the ratio is altered by statin treatment. Therefore, the main role for calculated LDL cholesterol is in assessment of suspected FH before treatment.
Non-HDL cholesterol represents the total sum of cholesterol in apoB-containing lipoproteins. This includes lipoprotein(a) (Lp[a]), which comprises a cholesterol-rich LDL particle with one molecule of apo B100 and an additional protein, apolipoprotein(a). Elevated Lp(a) is associated with increased risk of cardiovascular disease, particularly if levels exceed 50 mg/dL (500 mg/L or 125 nmol/L) where the risk of MI is increased two- to three-fold.11 The association between elevated Lp(a) and increased cardiovascular disease risk was continuous and did not depend on LDL cholesterol levels. On this basis, a position statement from the European Atherosclerosis Society has recommended screening for elevated Lp(a), with consideration of nicotinic acid (niacin) in addition to statins in patients with elevated levels, aiming for a Lp(a) level <50 mg/dl.11
There has been much debate concerning the role of other novel risk factors such as systemic inflammatory biomarkers in risk assessment. However, current evidence suggests that these add little and are not currently recommended.
Key messages
- Diagnosis of dyslipidaemia should involve a thorough clinical assessment. Secondary causes of dyslipidaemia should be excluded
- LDL cholesterol is important in the diagnosis of familial hypercholesterolaemia; apoB is diagnostic in mixed dyslipidaemia
- Measurement of lipoprotein(a) should be considered in patients with a personal or family history of coronary heart disease
- Systemic inflammatory risk markers add little to risk assessment
References
- Genest JJ Jr, Martin-Munley SS, McNamara JR et al. Familial lipoprotein disorders in patients with premature coronary artery disease. Circulation 1992;85:2025–33.
- Reiner Z, Catapano AL, De Backer G et al. ESC/EAS guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J 2011;32:1769–818. http://dx.doi.org/10.1093/eurheartj/ehr158
- Austin MA, Hutter CM, Zimmern RL, Humphries SE. Genetic causes of monogenic heterozygous familial hypercholesterolemia: a HuGE prevalence review. Am J Epidemiol 2004;160:407–20. http://dx.doi.org/10.1093/aje/kwh236
- National Collaborating Centre for Primary Care. CG71 Familial hypercholesterolaemia: full guideline. Identification and management of familial hypercholesterolaemia
(FH). August 2008. Available from: http://guidance.nice.org.uk/CG71/Guidance/pdf/English [accessed 13 December 2011]. - Soutar AK, Naoumova RP. Mechanisms of disease: genetic causes of familial hypercholesterolemia. Nat Clin Prac Cardiovasc Med 2007;4:214−25. http://dx.doi.org/10.1038/ncpcardio0836
- Civeira F, Castillo S, Alonso R et al.; Spanish Familial Hypercholesterolemia Group. Tendon xanthomas in familial hypercholesterolemia are associated with cardiovascular risk independently of the low-density lipoprotein receptor gene mutation. Arterioscler Thromb Vasc Biol 2005;25:1960–5. http://dx.doi.org/10.1161/01.ATV.0000177811.14176.2b
- Neil HA, Hammond T, Huxley R, Matthews DR, Humphries SE. Extent of underdiagnosis of familial hypercholesterolaemia in routine practice: prospective registry study. BMJ 2000;321:148. http://dx.doi.org/10.1136/bmj.321.7254.148
- Blom DJ, O’Neill FH, Marais AD. Screening for dysbetalipoproteinemia by plasma cholesterol and apolipoprotein B concentrations. Clin Chem 2005;51:904−07. http://dx.doi.org/10.1373/clinchem.2004.047001
- Christoffersen M, Frikke-Schmidt R, Schnohr P et al. Xanthelasmata, arcus corneae, and ischaemic vascular disease and death in general population: prospective cohort study. BMJ 2011;343:d5497. http://dx.doi: 10.1136/bmj.d5497.
- The Emerging Risk Factors Collaboration. Major lipids, apolipoproteins, and risk of vascular disease. JAMA 2009;302:1993–2000. http://dx.doi.org/10.1001/jama.2009.1619
- Nordestgaard BG, Chapman MJ, Ray K et al. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J 2010;31:2844–53. http://dx.doi.org/10.1093/eurheartj/ehq386
Case scenario
Differentiating familial causes of hypercholesterolaemia
 Derek, a 40-year-old former RAF engineer, was referred to the lipid clinic after recent admission to the Rapid Access Chest Pain Clinic with burning central chest pain (exercise electrocardiogram [ECG] was negative). Simvastatin was initiated by the clinician but Derek later stopped this due to severe headache and fatigue.
Derek, a 40-year-old former RAF engineer, was referred to the lipid clinic after recent admission to the Rapid Access Chest Pain Clinic with burning central chest pain (exercise electrocardiogram [ECG] was negative). Simvastatin was initiated by the clinician but Derek later stopped this due to severe headache and fatigue.
Derek was a non-smoker, physically active, a light drinker (4–6 units per week), was overweight (BMI 29.5 kg/m2) and had well-controlled blood pressure (136/84 mmHg), and normal renal, hepatic and thyroid function. Fasting glucose was 4.7 mmol/L. There was no family history of cardiovascular disease. However, on examination, there was xanthelasma together with elevated fasting total cholesterol (8.1 mmol/L), although triglycerides were near normal range (1.8 mmol/L). On a repeat fasting test one month later, both were higher (8.4 mmol/L total cholesterol and 3.4 mmol/L triglycerides). HDL cholesterol was within normal limits (1.4 mmol/L).
This case scenario highlights the variable phenotype of the lipid abnormality in combined dyslipidaemia and the importance of family history. The first profile is suggestive of possible familial hypercholesterolaemia, the second suggests that a diagnosis of combined dyslipidaemia typically associated with metabolic syndrome may be more likely. At least two lipid profiles are required to make a diagnosis.

