New high-sensitivity troponin assays will reduce the threshold for the diagnosis of myocardial infarction (MI), as specified in the 2012 third Universal Definition of MI. They will also allow earlier diagnosis of MI, but serial testing is required for adequate specificity. They convey prognostic information in both MI and in other acute conditions. Interpretation of troponin results must be in combination with a full assessment of the clinical context.
This review discusses these concepts and developments in this area.
Introduction
Myocardial infarction (MI) remains a common diagnosis and cause of death in the UK, despite sustained improvements in outcomes over more than a decade.1 Clarity in defining MI is important for both clinical diagnosis and research, but the way in which it is defined may significantly change patterns of diagnosis. The adoption of a troponin standard for the definition of MI in 2000 increased the incidence of MI by approximately 15%,2 and undoubtedly identified more high-risk patients for whom aggressive management would be beneficial. At the 2012 European Society of Cardiology (ESC) meeting the Universal Definition of MI was updated for the third time in a decade,3 and a major inclusion was the introduction of high-sensitivity cardiac troponin assays, which are again likely to change the rate of MI diagnosis. This review will discuss these new assays and their considerable potential to influence our practice.
1. Troponin testing is now highly sensitive
Troponin assays have developed with successive generations such that they can detect increasingly low levels of troponin.4 This has incrementally reduced the diagnostic cut-offs for the rule-out of MI from 0.5 µg/L in the first generation assays to the currently used levels of 0.05–0.1 µg/L (3rd generation), which are in widespread use today. However, the recent development of high-sensitivity troponin (hsTn) assays, which are in use in a few UK centres, can detect levels as low as 0.003 µg/L (3 ng/L). Of these, the high-sensitivity Roche Elecsys troponin T (hsTnT) assay is in use in our institutions, and has an upper limit of normal (99th centile) of 14 ng/L. This exquisitely sensitive assay removes the need to wait several hours after the onset of chest pain symptoms required by previous assays in order to reliably detect enough circulating troponin to confirm or exclude MI. It also means that the concept of a ‘negative’ troponin becomes relatively meaningless as troponin at some level can almost always be detected even in healthy patients. Intuitively, these concepts will have widespread clinical implications.
The diagnostic sensitivity of hsTn assays (ability to rule-out MI) are of the order of 90–95% when tested at the point of admission.5,6 When compared with older assays this is a marked improvement, but it still allows the potential for missing 5–10% of all MIs, with potentially adverse consequences for patient care. If, however, sampling is taken 3–6 hours after admission, the sensitivity is excellent at 99–100%.6 This is as expected for a more sensitive assay, as it is simply detecting the same troponin release at an earlier time than conventional assays. As a result, ESC guidelines recommend sampling at 3 hours after admission, with repeat sampling at 6 hours in patients still considered at risk,6 although there is some evidence to suggest that 3 hours may be too early in patients presenting early after pain.7 This will potentially have a large influence on the number of patients admitted to hospital to wait for troponin testing, and may have an even greater impact in low-risk populations, such as those tested in the Randomised Assessment of treatment using Panel Assay of Cardiac markers (RATPAC)8 and 2-Hour Accelerated Diagnostic Protocol to Assess Patients With Cheat Pain Symptoms Using Contemporary Troponins as the Only Biomarker (ADAPT)9 trials using 3rd generation troponin, where a 2-hour rule-out strategy may be used.
2. It is not as specific
The counterpoint to the excellent sensitivity of hsTn testing is a lower specificity (ability to rule-in MI). Although cardiac troponin is, by definition, completely specific for myocardial injury, it is not specific for the diagnosis of acute MI. This leads to a problem for hsTn where specificity has been reported to be 80–85%5 in comparison with figures of 97% for earlier generation troponins. This may have considerable impact on the utility of hsTn testing, as higher false-positive rates may lead to unnecessary subsequent invasive investigations, such as angiography. However, these lower specificities are based upon hsTn testing at much earlier time points and under-estimate the specificity of hsTn. In fact, if early hsTn testing is assessed against diagnoses made on the basis of serial hsTn testing, specificity is 92%.10
Despite taking these points into account, the lower specificity of hsTn testing remains a limitation in accurately diagnosing MI. To improve this, serial sampling is required – which is in fact necessary to satisfy the agreed criteria for a diagnosis of MI. The Universal Definition3 requires a change in troponin to be detected, although this has been frequently ignored in clinical practice, perhaps due to unwanted complexity or lack of general understanding of this concept. Sampling hsTn at admission and then a later time point allows calculation of the change between the two samples (known as the ‘delta’ value). If this is small it is considered to be a product of analytical or biological variation, whereas larger changes represent continuing troponin leak from damaged cells and, hence, suggest MI. In addition to this improved diagnostic accuracy, a proportion of patients will be ruled in as probably having an MI from the earliest sample, accelerating treatment.
The extent of change in troponin levels required to diagnose an MI is recommended by the ESC guidance as over a 20% relative change (in those patients with an elevated initial hsTn). However, this figure of 20% is based on research using older troponin assays, and although it is widely quoted in guidelines, there is evidence that for hsTnT testing in particular an absolute change of 7–9 ng/L provides better discrimination.11 A universal consensus is required for the use of delta values before wider adoption.
3. It is still not the only element of MI diagnosis
Many clinicians will agree that there is a problem in daily practice with ‘troponin-itis’ when any abnormal troponin result is automatically assumed to represent an MI. This problem will potentially worsen as our ability to detect much lower levels of troponin increases. hsTn is detectable in the majority of unwell patients, including those with sepsis or renal failure, which reflects low-level myocardial injury rather than MI.12 Elevated levels often represent myocardial injury induced by non-cardiac disease,3,6 or in some cases, troponin release will be due to a mismatch between myocardial blood supply and demand, resulting in a MI (defined as a type 2 MI3).
It is important to take into account the fundamentals of the history, examination and electrocardiogram (ECG) and these must be correlated with troponin results, as is clearly stated within the Universal Definition of MI.3 Importantly, the troponin assay is unable to exclude unstable angina, a high-risk clinical syndrome without MI (and with no troponin release), which requires careful clinical evaluation.
Our recognition of the limitation of troponin testing means that risk-scoring systems such as the Thrombolysis In Myocardial Infarction (TIMI) or Global Registry of Acute Coronary Events (GRACE) scores become important in the assessment of patients with chest pain. Strategies that combine risk scoring, ECG interpretation and troponin results (accelerated diagnostic pathways), have been recently tested in two large observational trials based in Australia and New Zealand. These showed that patients with a zero TIMI score, a normal ECG and normal troponin levels at 2 hours after admission had a 1% incidence of adverse events in the following 30 days. The authors suggest that this group of patients, which represented 10% of the population tested, were suitable for early discharge from hospital.9 These trials used 3rd generation troponin assays, and it is possible that hsTn would further improve discriminatory power. Further research is required to analyse the performance of hsTn assays in accelerated diagnostic pathways (see figure 1).
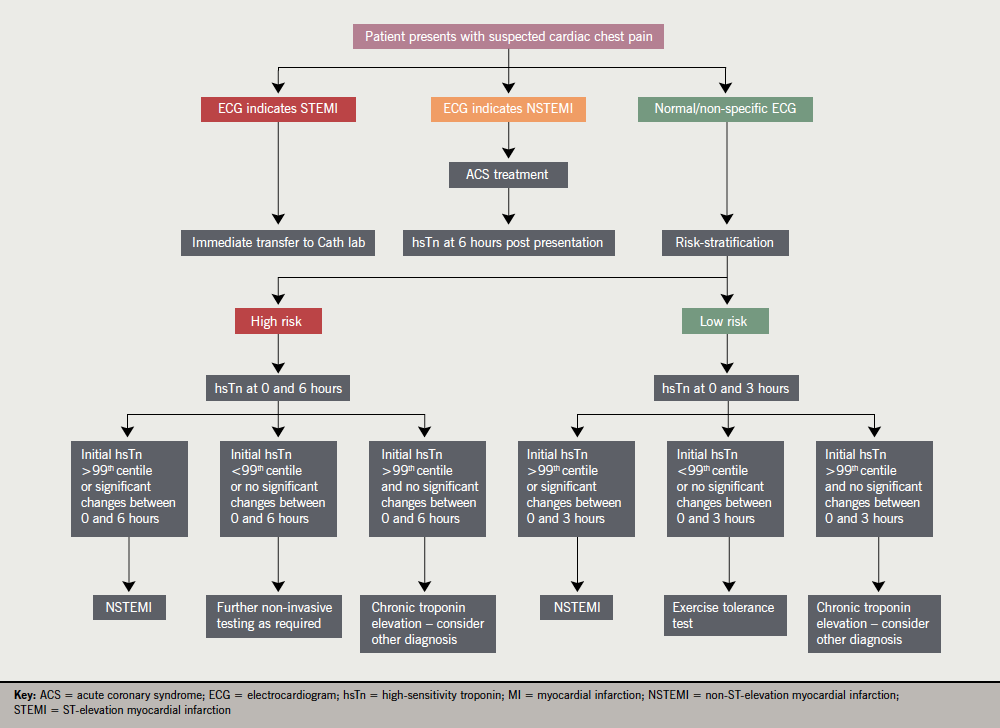
does not exclude unstable angina or ischaemic heart disease. After Collinson(12)
4. It is prognostic
Even when hsTn elevation is not caused by MI, it still provides important information. In every condition in which it has been assessed, elevated troponin levels correlate with an adverse prognosis (figure 2). Examples would include heart failure, atrial fibrillation, renal failure, pulmonary embolism, sepsis and surgery. These findings are not confined to acute illness but also apply to chronic stable disease. For example, in stable outpatients with risk factors for coronary disease, the level of hsTnT detected in stored blood samples correlated closely with prognosis, with the highest risk group, who had hsTnT of over 14 ng/L, having a four-times higher risk of death over a mean follow-up period of 9.4 years.13
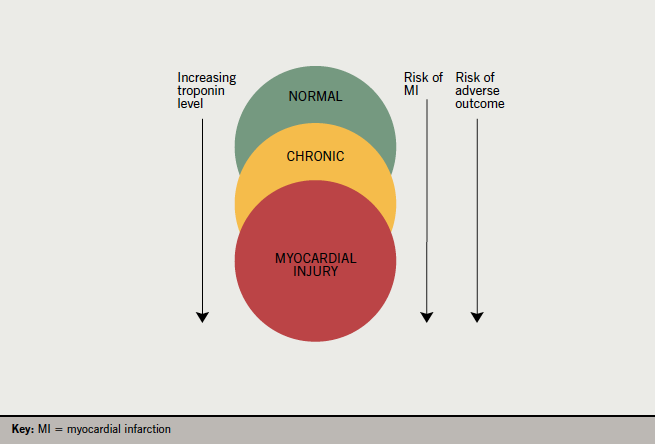
5. It has significant implications for MI diagnosis and management
The introduction of hsTn testing is already happening in many hospitals in the UK and will undoubtedly change management. Due to the increased sensitivity of these assays it is likely that the rate of MI diagnosis will increase. Some authors suggest that large increases in MI rates are possible, between 10%10 and 47%.14
However, it is not entirely clear what the clinical implications are in these patients with very low troponin elevations of less than 50 ng/L. One study showed that patients reclassified as MI had significantly better outcomes when clinicians acted on these results.15 However, this was based on a change of troponin threshold from 200 ng/L down to 50 ng/L, and hsTn tests measure levels down to a quarter of this level.
Use of hsTn is also almost certainly financially beneficial. An accurate diagnosis can be made at an earlier stage, potentially reducing length of stay. This should also increase the number of patients discharged on the same day as admission, which carries a tariff benefit in the National Health Service (NHS) system.16 A modelling study found that early hsTn testing was financially beneficial in almost all cases.17 It is likely that the next update of the UK National Institute for Health and Clinical Excellence (NICE) guidelines on the assessment and management of chest pain will assess hsTn testing.
6. Its use is still being refined
hsTn assays have only been available since 2010, and commercial availability is still limited to a small number of true high-sensitivity assays.6 Thus, it is still an area of very active research. While the current ESC guidelines recommend sampling at 3–6 hours after admission, it is likely that protocols will be developed to accelerate diagnosis further.
One group, based in Manchester, UK, investigated using the limit of detection of the hsTnT assay to identify a very-low-risk group. They found that a hsTnT level of less than 3 ng/L at presentation had a sensitivity to rule out MI of 99.8%, allowing early rule-out for up to a quarter of patients, but with a specificity of only 34% and the death of one patient in the early rule-out cohort.18 Another group used hsTnT sampling at admission and 1 hour later, and generated an algorithm that allowed the rule-in or rule-out of MI in up to 75% of patients tested.19 The remaining 25% of patients who did not fulfil rule-out or rule-in criteria required further troponin testing at 6 hours. Neither of these trials managed patients based on these results, and so these strategies are not yet ready for clinical use.
Further refinements in clinical use will likely be forthcoming in the future. It is already clear that ‘normal’ values for these assays are hard to derive. Normal, in a population of healthy blood donors, will not be the same as that in the older and generally sicker populations that present to hospital with symptoms of chest pain, and it is even conceivable that normal values need to be sex-specific.3 Certainly, the normal and ‘delta’ values need to be calculated for each assay individually.
Conclusion
Use of high-sensitivity troponin testing is likely to have a major positive influence on cardiology practice in the next few years, accelerating diagnosis and potentially improving outcomes. However, there is potential for confusion and increased workload if its introduction is not carefully managed and, in particular, is accompanied by a strong emphasis on concurrent clinical assessment. A consensus on the appropriate testing and assessment strategy will help reduce variations in practice and ensure clinicians are able to get the best out of these assays.
Conflict of interest
KG has received a grant from AstraZeneca to carry out research into hsTn assays and their clinical use.
Sources of support for research
JG and EC were undertaking a service improvement fellowship sponsored by NHS South Central. EC has received grant funding from the UK College of Emergency Medicine and Bournemouth University to carry out research into hsTn in the early rule-out of MI.
Key messages
- New high-sensitivity troponin assays will reduce the threshold for the diagnosis of myocardial infarction (MI)
- They will also allow earlier diagnosis of MI, but serial testing is required for adequate specificity
- They convey prognostic information in both MI and in other acute conditions
- Interpretation of troponin results must be in combination with a full assessment of the clinical context
References
- Smolina K, Wright FL, Rayner M, Goldacre MJ. Determinants of the decline in mortality from acute myocardial infarction in England between 2002 and 2010: linked national database study. BMJ 2012;344:d8059. http://dx.doi.org/10.1136/bmj.d8059
- Kavsak P, Macrae A, Lustig V et al. The impact of the ESC/ACC redefinition of myocardial infarction and new sensitive troponin assays on the frequency of acute myocardial infarction. Am Heart J 2006;152:118–25. http://dx.doi.org/10.1016/j.ahj.2005.09.022
- Thygesen K, Alpert JS, Jaffe AS et al. Third universal definition of myocardial infarction. Eur Heart J 2012;33:2551–67. http://dx.doi.org/10.1093/eurheartj/ehs184
- Jesse RL. On the relative value of an assay versus that of a test: a history of troponin for the diagnosis of myocardial infarction. J Am Coll Cardiol 2010;55:2125–8. http://dx.doi.org/10.1016/j.jacc.2010.03.014
- Reichlin T, Hochholzer W, Bassetti S et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med 2009;361:858–67. http://dx.doi.org/10.1056/NEJMoa0900428
- Thygesen K, Mair J, Giannitsis E et al. How to use high-sensitivity cardiac troponins in acute cardiac care. Eur Heart J 2012;33:2252–7. http://dx.doi.org/10.1093/eurheartj/ehs154
- Gamble J, Carlton E, Orr W, Greaves K. We have the guideline, now we need a consensus. A response to the ESC guidance on use of high-sensitivity cardiac troponins in acute cardiac care. Eur Heart J 2012;published online. Available from: http://eurheartj.oxfordjournals.org/content/33/18/2252/reply#ehj_el_1417
- Goodacre SW, Bradburn M, Cross E, Collinson P, Gray A, Hall AS. The randomised assessment of treatment using panel assay of cardiac markers (RATPAC) trial: a randomised controlled trial of point-of-care cardiac markers in the emergency department. Heart 2011;97:190–6. http://dx.doi.org/10.1136/hrt.2010.203166
- Than M, Cullen L, Aldous S et al. 2-hour accelerated diagnostic protocol to assess patients with chest pain symptoms using contemporary troponins as the only biomarker: the ADAPT trial. J Am Coll Cardiol 2012;59:2091–8. http://dx.doi.org/10.1016/j.jacc.2012.02.035
- Aldous SJ, Florkowski CM, Crozier IG, Than MP. The performance of high sensitivity troponin for the diagnosis of acute myocardial infarction is underestimated. Clin Chem Lab Med 2012;50:727–9. http://dx.doi.org/10.1515/cclm.2011.830
- Mueller M, Biener M, Vafaie M et al. Absolute and relative kinetic changes of high-sensitivity cardiac troponin T in acute coronary syndrome and in patients with increased troponin in the absence of acute coronary syndrome. Clin Chem 2012;58:209–18. http://dx.doi.org/10.1373/clinchem.2011.171827
- Collinson PO. Sensitive troponin assays. J Clin Pathol 2011;64:845–9. http://dx.doi.org/10.1136/jclinpath-2011-200164
- Saunders JT, Nambi V, De Lemos JA et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the atherosclerosis risk in communities study. Circulation 2011;123:1367–76. http://dx.doi.org/10.1136/jclinpath-2011-200164
- Mills NL, Lee KK, McAllister DA et al. Implications of lowering threshold of plasma troponin concentration in diagnosis of myocardial infarction: cohort study. BMJ 2012;344:e1533–e1533. http://dx.doi.org/10.1136/bmj.e1533
- Mills NL, Churchhouse AMD, Lee KK et al. Implementation of a sensitive troponin I assay and risk of recurrent myocardial infarction and death in patients with suspected acute coronary syndrome. JAMA 2011;305:1210–16. http://dx.doi.org/10.1001/jama.2011.338
- Department of Health. Confirmation of Payment by Results (PbR) arrangements for 2012–13. London: DoH, 2012. Available from: http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndG
uidance/DH_132654 - Thokala P, Goodacre SW, Collinson PO et al. Cost-effectiveness of presentation versus delayed troponin testing for acute myocardial infarction. Heart 2012;98:1498–503. http://dx.doi.org/10.1136/heartjnl-2012-302188
- Body R, Carley S, McDowell G et al. Rapid exclusion of acute myocardial infarction in patients with undetectable troponin using a high-sensitivity assay. J Am Coll Cardiol 2011;58:1332–9. http://dx.doi.org/10.1016/j.jacc.2011.06.026
- Reichlin T, Schindler C, Drexler B et al. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med 2012;172:1211–18. http://dx.doi.org/10.1001/archinternmed.2012.3698
