The Heart Rhythm Congress (HRC) remains the largest and fastest growing heart rhythm meeting in the UK, providing education and training to promote diversity and improved technology for all involved in the treatment of cardiac arrhythmia patients. We report highlights from this year’s meeting, HRC 2013, held in Birmingham from September 20–23, 2013.
What’s in a name
Last year’s meeting saw Heart Rhythm UK (HRUK) change its title to the British Heart Rhythm Society (BHRS). Explaining the change, BHRS President Elect Dr Nick Linker (South Tees Hospitals NHS Foundation Trust) said that this made things more flexible for the future, and will also avoid confusion with any other organisation using the abbreviation HRUK.
Ectopy in low- and high-risk patients

When are palpitations benign and when do they signal high risk? This thorny issue was addressed by Mrs Angela Hall, Lead Cardiac Rhythm Management (CRM) Specialist Nurse (James Cook University Hospital, Middlesbrough) and Dr Elijah Behr (St George’s Hospital, London) who looked at how to predict high risk in patients with palpitations.
Speaking to the BJC, Mrs Hall said: “Palpitations are a common presentation and a frequent reason for cardiology referral. They are, however, often benign, with less than half of patients suffering from an arrhythmia. Many patients will suffer from low-risk palpitations, however, this can still cause considerable stress and anxiety to both patients and healthcare professionals. The key to assessment is identifying patients with a significant heart rhythm abnormality at risk of adverse outcome that may require treatment.”
She described how such patients could be identified: “This can be achieved by carrying out a detailed history, electrocardiogram (ECG), echocardiogram, and clinical examination. Investigating symptoms in the form of ambulatory monitoring and event recorders will provide symptom correlation, which is important in reassuring patients of an underlying cause. Vital to the assessment is ruling out any structural heart abnormality and significant family history. Recording an ECG and acting on abnormal findings is paramount. An explanation of the mechanism behind the palpitations, reassurance and lifestyle advice is often all that is required in terms of management for low-risk palpitations. In some cases medical management may also be considered to enable the patient’s symptoms to be managed at an acceptable level.”

How to predict high risk in patients with palpitations, was addressed by Dr Behr. First, what sort of palpitations are they? Are there uncomplicated ectopics, characterised by irregular, forceful beats, he asked? High-risk markers include known cardiac (including structural) or genetic disease, or family history of sudden cardiac death (SCD). Similarly, is there pre-syncope, or exertionally induced syncope? “Youth is not a reassuring factor,” he said.
Investigations include a 12-lead ECG, echocardiogram, and cardiac magnetic resonance imaging (MRI) for structural heart disease, possibly with gadolinium contrast to detect scarring. Also, an exercise ECG may be revealing, along with Holter monitoring, or implantable loop recorder, and, ultimately, an electrophysiological (EP) study, “the refuge of the undiagnosed”.
Non-sustained ventricular tachycardia (VT) on Holter monitoring in a young patient with cardiomyopathy can be ominous. Sustained VT is also a potential high-risk marker, especially in the presence of reduced ejection fraction, warranting referral. Atrial fibrillation (AF) is a “high-risk arrhythmia”, which doubles risk of mortality. If a patient is on anti-arrhythmic therapy they should be monitored, as such treatment can be pro-arrhythmic.
Exercise

Chairman Dr Matt Fay (Westcliffe Medical Centre, Yorkshire) suggested that “cardiology involves ongoing layers of risk stratification”. Not all patients may require an echo, sometimes B-type natriuretic peptide (BNP) testing may help. Questions arose on what advice should be offered regarding exercise in ‘at-risk’ patients. “These are best assessed on a case-by-case basis,” and it depends on the aetiology (phenotype), said Dr Fay.
If, for example, the arrhythmia is adrenergically driven, then sports exercise is inadvisable. Arrhythmogenic right ventricular cardiomyopathy (ARVC) may be partly induced by exercise. Deaths in Brugada syndrome, however, characteristically occur at rest, and exercise is not necessarily limited. Some long QT syndrome patients are more at risk than others and exercise is often restricted, although the evidence base is not great. Better rapport is needed between secondary and tertiary cardiac centres, particularly where cascade screening of families is concerned. A meeting following the forthcoming Association of Inherited Conditions (AICC) will address aspects of Specialist Commissioning, funding for such investigations. Dr Fay concluded that SCD in an athlete is, fortunately, “a rare but highly visible tragedy”.1
Dr Fay also discussed results from the Birmingham group in the SAFE (Saline versus Albumin Fluid Evaluation) study,2 from which “we know that one in 10 of the over 65 population will have an irregular pulse on clinical examination – however, the minority have AF and many of the others have ectopy. This is a large population for primary care to risk stratify and some direction on when further expert advice is required would assist the primary care clinicians”.
Young Investigator’s Award
Molecular autopsy was the topic addressed by Dr Hari Raju (St George’s Hospital, London) who was the winner of the Young Investigator’s Award (Basic Science). He describes this fast-developing field (see box on molecular autopsy).
The other Young Investigator’s Award (Clinical Science) was awarded to Dr Fozia Ahmed (Manchester Heart Centre) for his work on the clinical utility of fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography as a diagnostic tool in patients with non-valvular cardiac device-related infection.

What is a molecular autopsy? The HRC Young Investigator Award
Premature sudden deaths that remain unexplained, despite complete autopsy including toxicological examination, are referred to as sudden arrhythmic death syndrome (SADS). Approximately 500 annual SADS deaths occur in the UK, and half are associated with previously undiagnosed inherited arrhythmia syndromes, such as the long QT (LQT), Brugada (BrS) and catecholaminergic polymorphic ventricular tachycardia (CPVT) syndromes, with phenotypically mild cardiomyopathies contributing a small minority.3 This is evidenced by the familial diagnoses identified on cardiological investigation of first-degree blood relatives; evaluation of blood relatives is advocated following a SADS death in Chapter 8 of the National Service Framework (NSF) to identify surviving family members who remain at risk.
Given the genetic aetiology of these conditions, molecular autopsy, or direct genetic evaluation of the SADS case provides an alternative avenue to establish post-mortem diagnoses. However, the differential diagnosis includes multiple conditions, each of which is associated with rare mutations affecting one of many risk-genes. Moreover, modern science has not yet identified all risk-genes for these conditions, thereby limiting the yield further.
Thus, despite a recent joint European and US consensus statement acknowledging the utility of molecular autopsy as a class 2A recommendation,4 its widespread adoption in the UK is limited by the cost and workload implications of offering such investigation, and the inability to target specific risk-genes. To our knowledge, neither the coronial services, nor the NHS, are offering routine molecular autopsy in the UK, except in the presence of a definitive familial phenotype, at present.
Next-generation sequencing (NGS) is an emerging research technology that offers the potential for the parallel study of large multi-gene panels, in many cases, at once. NGS is frequently utilised for whole genome and whole exome sequencing, although smaller gene panels can be equally cost-effective. Our study utilised an NGS panel of five major LQT and BrS risk-genes, in addition to the major CPVT risk-gene. Mutation-detection was optimised by post-sequencing bioinformatic adjustments, with subsequent replication in a second patient series, demonstrating validity of the molecular autopsy findings.
Overall, we demonstrated a molecular diagnostic yield of up to 13% (n=20/151), lower than other large published series,3 but complementary to familial evaluation. We believe that NGS panels may facilitate establishment of genetic testing at lower cost with higher throughput as part of a tiered molecular autopsy strategy in SADS.
NOACs in DC cardioversion
There is considerable interest in the role of the novel oral anticoagulants (NOACs) in direct current cardioversion (DCCV) of AF compared with warfarin. Patients often struggle to maintain therapeutic international normalised ratio (INR) leading to rescheduling of their DCCV appointments, which is frequently very inconvenient.
Dr Wai Kah Choo and colleagues (Raigmore Hospital, Inverness) presented data from their nurse-led DCCV clinic, which was set up in 2006, and replaced warfarin with dabigatran where possible, from December 2011.
They evaluated 242 DCCVs, performed in 193 patients over a 36-month period. Patients were divided into two cohorts. Cohort A included cases in the 22-month period prior to the introduction of dabigatran. Cohort B included cases in the 14-month period after the introduction of dabigatran. In cohort B, 48.4% received dabigatran; the other patients were established and stable on warfarin or had reasons for taking warfarin (e.g. metal heart valve).
They found a significantly larger number of patients from cohort A were rescheduled due to subtherapeutic INRs compared with cohort B (42% vs. 15%, p<0.001). Those who received dabigatran also had significantly lower rates of rescheduling compared with those who received warfarin (9.7% vs. 34.4%, p<0.001). The length of time between initial clinic assessment and DCCV was 22 days shorter in patients taking dabigatran compared with warfarin (p=0.0015). Outcomes in achieving and maintaining sinus rhythm were comparable in both cohorts and anticoagulants (p>0.05). There were two cases of transient ischaemic attack (TIA) in the warfarin group, both within therapeutic INR range. There was one case of TIA in the dabigatran group.
The authors conclude that use of dabigatran in patients undergoing DCCV reduced rescheduling and improved efficiency compared to warfarin. Dabigatran may bring significant cost savings, if results are extrapolated to the rest of the country, the authors conclude. The full paper for this study is published in this issue (doi: 10.5837/bjc.2014.002).
Anticoagulate, yes or no?
Dr Matt Fay provided insight into a field that warrants the view, “complacency about AF-related stroke is not acceptable”. AF-related strokes are typically more severe, and warfarin has been shown to consistently reduce the risk of stroke or systemic embolism by about two thirds compared with no treatment, and by 30–40% compared with aspirin in high-risk AF patients. The CHADS2 score system is a simple way to assess risk, but when compared with the CHADS2 score, CHA2DS2-VASc performs better in predicting patients at high risk; and those categorised as low risk by CHA2DS2-VASc are truly at low risk for thromboembolism.
About 5% of the population over 65 years has AF and even subclinical (asymptomatic) AF increases stroke risk, while paroxysmal AF appears to carry similar risk to persistent/permanent AF. Yet there are barriers to anticoagulation – including age, falls, dementia, poor INR control, permanent versus sustained AF – which should not be barriers, and it is often the patients who are denied anticoagulation who have the most to gain. It has been shown, for example, that the benefits of anticoagulation outweigh the risk of falls.
Dr Fay asked why with such an evidence base, adoption of stroke prevention strategies in this population remains inconsistent. After looking at the evidence for level of risk for intervention, paroxysmal compared with consistent AF, and risks of falls as barriers – among other topics – he ended with a reminder: “If the profession does not resolve this issue, then 5,000–10,000 people will suffer a possibly avoidable AF-related stroke each year.”
What are the pros and cons of the NOACs? Clearly they are convenient, and have a predictable and rapid onset/offset. Conversely, there are no reversal agents, no routine monitoring (anticoagulation assessment), and there are risks associated with missed dosing. Caution is required in relation to renal dysfunction, although cost may be the paramount issue. The NOACs are “very cost effective but not affordable in an NHS without very much money”, Dr Fay contends. However, as data emerge, showing benefit with the NOACs, “these are all part of the solution”.
AF: ablate, medicate or leave alone?

This intriguing topic was addressed by Dr Tim Betts (John Radcliffe Hospital, Oxford). AF accounts for about 1.1–3.6% of emergency department (ED) visits. Just over 3% of these patients are haemodynamically unstable. There is a 48-hour ‘window’ for cardioversion and after this there is an increased thromboembolic risk. There is a high rate of spontaneous reversion to sinus rhythm, however, there is a choice between rhythm or rate control. While flecainide has high cardioversion rates between 2 and 8 hours, it can be pro-arrhythmic and should be only used in structurally normal hearts with a low likelihood of ischaemic heart disease. Digoxin is “the least effective rate controller”, and beta blockers and rate-lowering calcium antagonists are the drugs of choice for rate control. There is no role for amiodarone, he said, which takes 24 hours to work as a rate controller and is no better than placebo at acute pharmacological conversion in the ED.
If sinus rhythm is not restored, what is the next outpatient option? The goals are to address the stroke risk, to reduce symptoms and improve quality of life and to avoid tachycardia-induced cardiomyopathy, which can occur with persistent AF. The ‘pill in the pocket’ approach with flecainide is useful in paroxysmal AF, the downside being that AF may become flutter.
The treatment strategy depends on the relative contribution of rapid rate and loss of atrial contractility. “Although long-term sinus rhythm is very difficult to achieve, if successful it’s better than AF”. DCCV may be worth undertaking as a ‘diagnostic tool’ to see whether the patient differentiates between AF and sinus rhythm. AF ablation has been practiced for many years. It takes 2–3 hours with a success rate of about 80–90%, but the Achilles heel is that one in three patients “need more than one go”. Also, the only role for ablation is in symptomatic treatment – “ablation has not yet been shown to reduce stroke risk”. While it is useful to see patients through all the ‘drug steps’ first, ablation is an increasingly popular option, especially in younger and more active patients.
Preventing deterioration in LVSD

Dr Andrew Turley (James Cook University Hospital, Middlesbrough) reviewed how preventing deterioration in left ventricular systolic dysfunction (LVSD) can be achieved with interventions such as cardiac resynchronisation therapy (CRT). While not much has changed in considering implantable cardioverter defibrillators (ICDs), there has been a big evolution in who qualifies for CRT. And the CRT guidelines are “evolving rapidly”, so much so that the NICE (National Institute for Health and Care Excellence) guidelines are now significantly outdated (although may have a new iteration in the spring). Evolving indications include use in patients with AF, non-left bundle branch block, bradycardic pacing indications (irrespective of QRS duration), and minimally symptomatic patients (NYHA I/II) with a broad QRS complex.
The ECHO CRT (Echocardiography Guided Cardiac Resynchronisation Therapy) study was stopped for futility, but the study did reveal that if the QRS is less than 130 ms, then “don’t bother with CRT”, Dr Turley advised. CRT does not improve clinical outcome in patients with a QRS <130 ms.
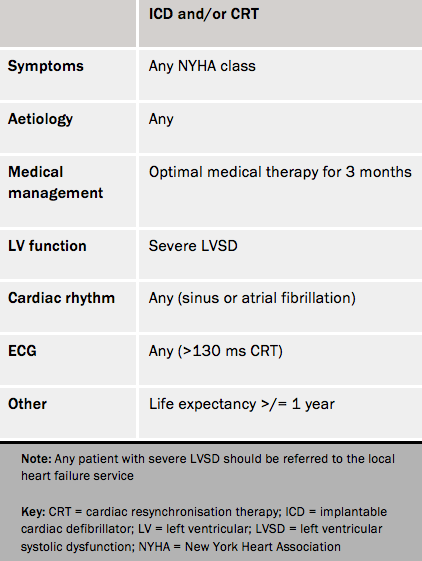
Within the UK, implant rates for life-saving cardiac devices (i.e. ICD and CRT) remain among the lowest in Europe. Severe LVSD should be an automatic referral for consideration of an ICD device and/or CRT. Useful online resources for guidance include www.arrhythmiaalliance.org.uk and www.screenlinkcalculator.com. Table 1 shows the North of England Cardiovascular Network referral criteria for ICD/CRT
The next Heart Rhythm Congress will be held at the International Convention Centre, Birmingham from October 5–8 2014. For more information, please visit http://
www.heartrhythmcongress.com/
Acknowlegement
We thank the following, who all contributed to this article:
- Dr Elijah Behr (St George’s Hospital, London)
- Dr Tim Betts (John Radcliffe Hospital, Oxford)
- Dr Wai Kah Choo (Raigmore Hospital, Inverness)
- Dr Matt Fay (Westcliffe Medical Centre, Yorkshire)
- Ms Angela Hall (James Cook University Hospital, Middlesbrough)
- Dr Hari Raju (St George’s Hospital, London)
- Dr Andrew Turley (James Cook University Hospital, Middlesbrough)
References
1. Chandra N, Bastienen R, Papadakis M, Sharma S. Sudden cardiac death in young athletes. J Am Coll Cardiol 2013;61:1027–40. http://dx.doi.org/10.1016/j.jacc.2012.08.1032
2. Mant J, Fitzmaurice DA, Hobbs FDR, et al. Accuracy of diagnosing atrial fibrillation on electrocardiogram by primary care practitioners and interpretative diagnostic software: analysis of data from screening for atrial fibrillation in the elderly (SAFE) trial. Br Med J 2007;335:380. http://dx.doi.org/10.1136/bmj.39227.551713.AE
3. Raju H, Behr ER. Unexplained sudden death, focussing on genetics and family phenotyping. Curr Opin Cardiol 2013;28:19–25. http://dx.doi.org/10.1097/HCO.0b013e32835b0a9e
4. Priori SG, Wilde AA, Horie M, et al. HRS/EHRA/APHRS Expert Consensus Statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Heart Rhythm 2013;29:e75–e106. http://dx.doi.org/10.1016/j.hrthm.2013.05.014
