The European Medicines Agency (EMA) has mandated that patients treated with dronedarone have regular monitoring. An arrhythmia specialist nurse (ASN) took over the care of patients on dronedarone in 2012.
Patients on dronedarone were identified from hospital notes and pharmacy records. Adherence to EMA guidelines on monitoring before and after the appointment of an ASN were compared. In 112 patients on dronedarone in the year prior to the appointment of an ASN, only 478 of the 1,275 (37%) required tests were actually done. With the ASN, 382 of 422 (92%) tests in 53 patients were performed. This was significantly better (p<0.001). Dronedarone was more likely to be stopped due to contraindications (p<0.017) prior to the appointment of ASN, but afterwards was more likely to be stopped due to side effects (p<0.001).
The ASN significantly improved adherence to EMA-mandated monitoring in patients on dronedarone. Involvement of an ASN had no overall impact on the likelihood of dronedarone being stopped. Patients were more likely to have the drug stopped due to side effects, and were less likely to stop for safety reasons. ASN care is superior to conventional follow-up, and is the gold standard for patients treated with dronedarone.
Introduction
Atrial fibrillation is the most common arrhythmia, affecting 1–2% of the population.1 It is associated with an increased risk of stroke and death, heart failure, reduction in quality of life, mental health problems and cognitive impairment.2 Hospitalisation is common and costly.3
Dronedarone was first approved by the National Institute for Health and Care Excellence (NICE) in April 20104 for atrial fibrillation rhythm control, but following two fatal cases of liver toxicity it is contraindicated in patients with liver dysfunction, a creatinine clearance (CrCl) ≤30 ml/min, in permanent atrial fibrillation or congestive heart failure; and should be discontinued in patients who develop these. The European Medicines Agency (EMA), in the Summary of Product Characteristics (SPC) for dronedarone,5 recommended patients should be carefully monitored and supervised by a specialist. Monitoring of liver function should be performed prior to initiation, at one week and monthly up to six months, and at nine and 12 months. Renal function should be tested one-week post-initiation and at regular intervals and electrocardiograms (ECGs) recorded six monthly. This monitoring can be difficult to perform and coordinate, and in October 2012 an arrhythmia specialist nurse (ASN) was appointed by East Sussex Healthcare NHS Trust to oversee this. Required blood tests were arranged with local GPs and coordinated by the ASN. In this paper, adherence to the stipulated schedule has been compared before and after this appointment.
Methods
Data were collected from a single NHS trust between 1 October 2011 and 1 October 2013. Patients started on dronedarone were identified from hospital pharmacy records. Compliance with monitoring was assessed from medical records, and date of cessation noted, where relevant. Data were censored at one year after dronedarone initiation, or at the time of study completion in October 2013, whichever was earlier. Data were stratified according to whether patients started dronedarone prior to, or after the appointment of an ASN. Baseline data, such as gender, age, medical history, echocardiogram findings and previous anti-arrhythmic drug use, were also collected.
Descriptive data were presented and compared in the standard manner. Proportions of tests per patient were compared. Survival analyses were performed for time to first failure to follow dronedarone treatment guidelines, and time to cessation of dronedarone treatment. Comparisons are reported as significant for a two-sided p value of <0.05, and stratified by ASN involvement.
Results
Characteristics of patients taking dronedarone
There were 112 patients identified as having started dronedarone prior to the appointment of the ASN, with 53 patients afterwards. There was no significant difference in age, pre-existing hypertension, thyroid dysfunction, diabetes or prior class I, II or IV anti-arrhythmic treatment (table 1).
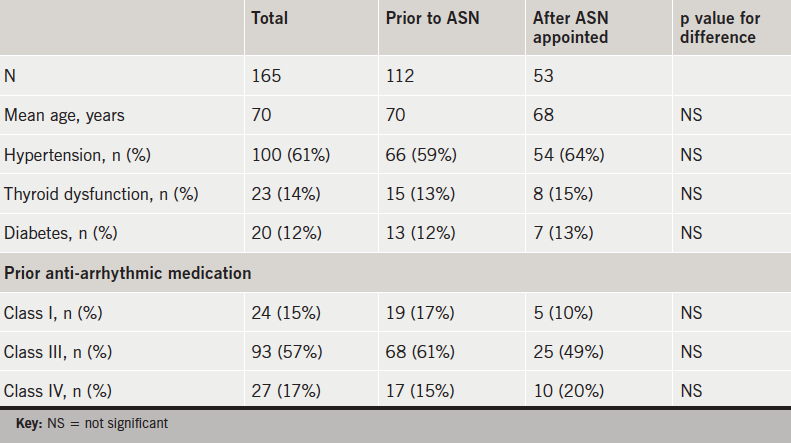
There was a significantly longer duration of follow-up (mean 360 vs. 295 days, p<0.001) for those starting dronedarone prior to ASN.
Adherence to recommended monitoring
There were 112 patients commenced on dronedarone prior to the appointment of an ASN. SPC recommendations required 1,275 tests and 478 (37%) were actually performed.
After the appointment of an ASN, 53 patients commenced dronedarone between October 2012 and September 2013. There were 422 tests required by the SPC and 382 were undertaken (92%); a significantly higher number (p<0.001) when compared with the period prior to ASN.
Those starting dronedarone after the ASN was appointed had a significantly longer duration of uninterrupted appropriate monitoring (p<0.001). Figure 1 displays a Kaplan-Meier survival curve, censoring follow-up at study termination or dronedarone cessation.

Dronedarone cessation
Of 165 patients starting dronedarone, 75 (45%) stopped during the study period, and this was more prompt following ASN appointment.
Reasons for cessation are shown in table 2. Prior to the ASN, patients were significantly more likely to stop due to contraindications as per SPC; most commonly non-paroxysmal atrial fibrillation. After the ASN was appointed, patients were significantly more likely to stop due to side effects. Drug-related effects were significantly more likely to be recorded after the ASN was appointed.

Despite concerns regarding liver function, only one patient experienced deterioration in liver function (peak alanine aminotransferase [ALT] 154 IU/L). This was during a hospital admission for pneumonia treated with high-dose intravenous antibiotics. Liver function returned to normal and he remains on dronedarone.
Six patients experienced a rise in potassium levels (peak 5.8 mmol/L) around month one of drug therapy. These resolved in all patients when tests were repeated.
Creatinine levels are known to rise in the early stages of drug therapy with dronedarone,5 and this was noted in seven patients. Levels returned to normal in four patients, but remained elevated in three cases. Peak levels were 187, 214 and 122 µmol/L. In the first two patients, there was a history of renal problems and left ventricular dysfunction; one patient discontinued therapy due to oedema and shortness of breath, but the other continues on therapy with close monitoring. The third patient had no history of renal problems and normal left ventricular function but is no longer taking dronedarone due to oedema and shortness of breath.
Of note, the other four patients who discontinued therapy due to oedema and shortness of breath did not have any change to renal function.
Discussion
Prior to the appointment of an ASN, compliance with recommendations for the monitoring of dronedarone was low. The rate of compliance markedly increased with the appointment of an ASN, from 37% to 92%. The trust covers a rural area with many patients in the study living long distances from a hospital (longest 51 miles). Cooperation between primary and secondary care is essential, and ASNs are ideally placed to provide this link. The EMA guidance for dronedarone monitoring is mandatory and these data confirm that an ASN is an effective way of complying.
Revised marketing authorisations have made the use of dronedarone more complicated, but this is not a disadvantage when supervised by an ASN. In practice, patients are reassured by the knowledge that there is a designated person overseeing their results and that anomalies can be identified quickly. Patients need careful education to encourage them to report any symptoms of heart or liver failure while taking the drug,6 and to comply with monitoring.
Our data indicate that concerns regarding the effect of dronedarone on liver function may be exaggerated. The one patient who had an elevation in ALT was treated for an infection with high-dose antibiotics and has now fully recovered. Renal function has required close monitoring in some patients. There were some instances of transient hyperkalaemia observed; most likely due to haemolysis.
Most patients who required dronedarone cessation did not have blood test abnormalities. This underlines the importance of an appropriately trained professional having regular clinical contact with patients.
There was no significant difference in rates of therapy discontinuation before and after the appointment of the ASN, but the reasons for dronedarone cessation were markedly different. Prior to the ASN, a large proportion of patients stopped dronedarone because of SPC violations. Involvement of an ASN dramatically reduced the likelihood of this highly undesirable situation occurring. Instead, patients reported side effects from dronedarone to the ASN, who could respond appropriately and quickly; sparing anxiety and distress.
Conflict of interest
MA: post funded by Sanofi. RG, SF, CS, NS: none declared..
Key messages
- Monitoring of dronedarone is complex
- The involvement of an arrhythmia specialist nurse improves adherence to mandatory monitoring and the response to patient side-effects
References
1. Stewart S, Hart CL, Hole DJ, McMurray JJ. Populations prevalence, incidence and predictors of atrial fibrillation in the Renfrew/Paisley study. Heart 2001;86:516–21. http://dx.doi.org/10.1136/heart.86.5.516
2. Stewart S, Hart CL, Hole DJ, McMurray JJ. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med 2001;113:359–64.http://dx.doi.org/10.1016/S0002-9343(02)01236-6
3. The Office of Health Economics. Estimating the direct costs of atrial fibrillation to the NHS in the constituent countries of the UK and at SHA level in England. Office of Health Economics, 2009.
4. National Institute for Health and Care Excellence. Atrial fibrillation – dronedarone: final appraisal determination. London: NICE, 2010. Available from: http://www.nice.org.uk/guidance/ta197/resources/atrial-fibrillation-dronedarone-final-appraisal-determination
5. European Medicines Agency. MULTAQ 400 mg film-coated tablets. Summary of Product Characteristics. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001043/WC500044534.pdf
6. Camm AJ, Savelieva I. Dronedarone for the treatment of non-permanent atrial fibrillation: National Institute for Health and Clinical Excellence guidance. Heart 2013;99:1476–80. http://dx.doi.org/10.1136/heartjnl-3013-303863
