Correspondence from the world of cardiology
Dear Sirs,
We read with interest Diana Gorog’s recent article on the uptake of non-vitamin K oral anticoagulants (NOACS) in the UK.1 She drew attention to the slow uptake of these agents in the UK as opposed to many countries in Europe, and certainly the USA, and to the role that local medicines management committees (MMCs) may play in this. While the National Institute for Health and Care Excellence (NICE) guidance regarding all three NOACs available in the UK (apixaban, dabigatran and rivaroxaban) is that they should be available as an option for stroke prevention in non-valvular atrial fibrillation (AF), many MMCs in the UK have sought to control prescriptions of the new agents by issuing policies which keep warfarin first line, and reserve NOACs for situations where warfarin is unsuitable.
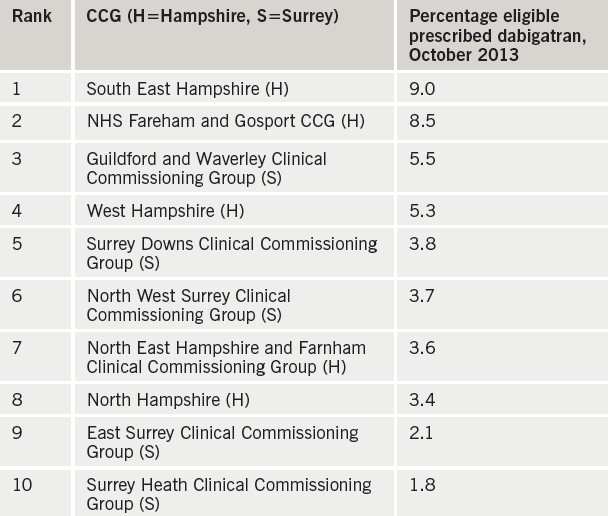
As part of a NICE scholarship project, we sought to collect further information on the influence of these restrictive local prescribing policies on the prescribing patterns of doctors. The focus of the project was on the Surrey/Hampshire area: this area was chosen as particularly illuminating, as the two neighbouring counties had different anticoagulant prescribing policies. Clinical Commissioning Groups (CCGs) in Surrey followed the policy of the Surrey Prescribing Clinical Network, which states: “Patients with a CHA2DS2-VASc score ≥2 or CHA2DS2-VASc score 1 and considered high risk should be initiated on warfarin in the first instance, unless contraindicated. Warfarin remains the agent of choice for the prevention of stroke and systemic embolism in AF”.
In Hampshire, on the other hand, CCGs largely followed the policy of Basingstoke, Southampton and Winchester District Prescribing Committee. This did not restrict clinician choice, but simply stated NICE’s position that new anticoagulants should be considered as an option. Particularly significantly, it stated: “Prescribers may wish to consider if newly diagnosed patients are suitable for the new OACs in the first instance”.
We focused on one of the NOACs – dabigatran – and compared prescribing rates in the five Hampshire and five Surrey CCGs. Data on number of prescriptions was obtained from dabigatran’s manufacturer, Boehringer Ingelheim; this was combined with CCG population numbers (Office for National Statistics data2) and AF prevalence (Health and Social Care Information Centre data3) to give an estimate of the population eligible for anticoagulation. Significantly, the top two CCGs for dabigatran prescribing were both in Hampshire, and the bottom two both in Surrey (see table 1).
We then sought further evidence for the influence of local prescribing policies by surveying 120 primary care prescribers in the Surrey/ Hampshire area. 33% responded; two thirds of respondents felt that their prescribing of dabigatran was influenced by financial pressure from their local CCG. Interestingly, however, there was no difference between Surrey and Hampshire prescribers in their answers to this question. We also asked respondents to pick the factor which had the strongest influence on their decision making regarding dabigatran prescribing. Replies showed an even split between NICE guidance, financial constraints, safety concerns and lack of familiarity (see figure 1).

We concluded that local prescribing policies do have an effect on prescribing habits, but that they are only one of a number of factors which influence prescribers when they are selecting an anticoagulant. NICE guidance clearly ranks among the most important of these factors, which is in agreement with data published recently in this journal by Campbell et al, suggesting that NOAC prescribing in the UK has increased since the publication of the revised NICE guidance on atrial fibrillation in 2014.4 This guidance emphasises the importance of anticoagulation for all but the lowest risk patients, and indicates that vitamin K antagonists and NOACs should be considered as equally valid options.5
As an aside, we also collected data on prescription rates for all anticoagulants, including warfarin, over the year from November 2012 to October 2013; we found a steady increase over time in prescriptions for all the anticoagulants. This confirms the encouraging data from registries such as PREFER in AF,6 which suggest that, regardless of anticoagulant used, increasing numbers of patients in the UK with AF who are at risk of stroke are being offered the protection of anticoagulation.
Acknowledgements
We thank Dr C Hendry, Royal Surrey County Hospital (Project Supervisor); Mr B White, Nottingham University Hospital (NICE Mentor); and Mr F Ratcliff, Boehringer Ingelheim.
Sources of support
This work was undertaken as part of National Institute for Health and Care Excellence Scholarship scheme 2013–2014.
Conflict of interest
None declared.
Dr Matthew Rogers
Haematology SpR
Royal Marsden Hospital, Sutton, Surrey SM2 5PT
Reference
1. Gorog D. Rivaroxaban in nonvalvular AF – UK experience in perspective. Br J Cardiol 2014;21 (suppl 1):S1–S11.
2. Annual Mid-year Population Estimates for Clinical Commissioning Groups, 2011. Office for National Statistics, August 2013. http://www.ons.gov.uk/ons/ publications/re-reference-tables. html?edition=tcm%3A77- 318167#tab-all-tables. Accessed July 2015.
3. Quality and Outcomes Framework, 2011–12, PCT level. Health and social care information centre, published October 2012. http://www.hscic.gov. uk/catalogue/PUB08722. Accessed July 2015.
4. Cowan C, Fay M, Maskrey N. The new NICE AF guideline and NOACs: a response. Br J Cardiol 2015;22:53– 5. http://dx.doi.org/10.5837/ bjc.2015.019
5. National Institute for Health and Care Excellence. Atrial fibrillation: the management of atrial fibrillation. CG180. London: NICE, June 2014. Available from: http://www.nice.org.uk/ guidance/cg180
6. Kirchhof P, Ammentorp B, Darius H et al. Management of atrial fibrillation in seven European countries after the publication of the 2010 ESC Guidelines on atrial fibrillation: primary results of the PREvention oF thromboemolic events – European Registry in Atrial Fibrillation (PREFER in AF). Europace 2014;16:6–14. http://dx.doi.org/10.1093/europace/eut263
