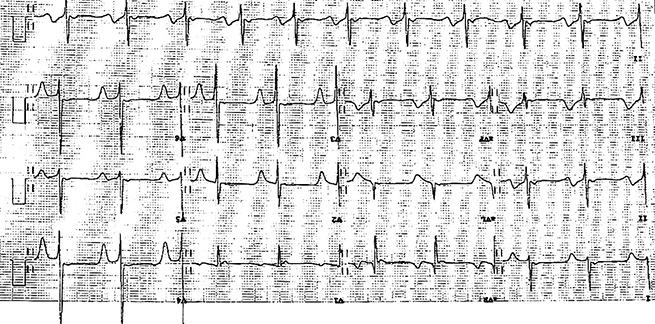
A sixty-two-year-old asymptomatic man presented for a routine insurance medical. He had no previous cardiac history, nor any significant cardiac risk factors. His examination was normal. His electrocardiogram (ECG), however, was noted to be significantly abnormal, with deep anterior T-wave inversion in the precordial leads (figure 1). Given this abnormality and the potential differential diagnoses, a cardiovascular magnetic resonance (CMR) (Siemens Aera 1.5 T) with regadenosine stress perfusion was performed and images analysed using CMR 42 software (Circle CVI, Calgary).
The CMR demonstrated marked asymmetrical hypertrophy of the anterior and anteroseptal walls at basal and mid-ventricular level. The peak basal wall thickness was 25 mm. The reference wall thickness was 7–9 mm. There was also apical circumferential hypertrophy with a peak apical wall thickness of 11 mm and systolic obliteration from distal left ventricle (LV) through to the apex. Perfusion was performed with regadenosine stress. There was severe microvascular ischaemia in a near circumferential pattern basally, and marked ischaemia in the maximally hypertrophied anterior and anteroseptal segment. At apical level there was circumferential ischaemia (figure 2). Late gadolinium images demonstrated extensive replacement fibrosis in the maximally hypertrophied anterior and anteroseptal walls at basal and mid-ventricular level.
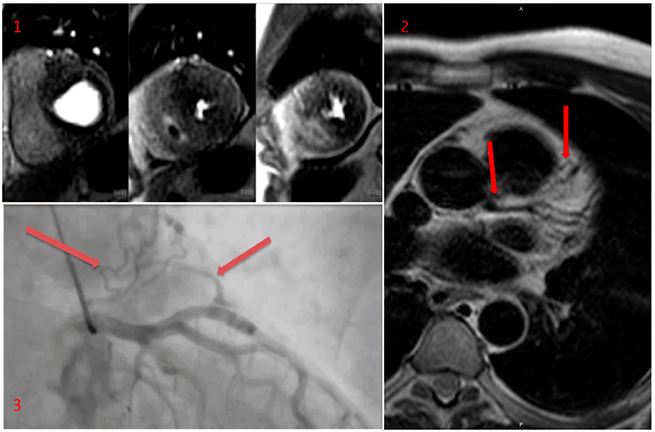
T2-weighted turbo-spin echo (TSE) images were acquired from base to apex in transaxial views based on a suggestion of coronary fistulae and demonstrated a probable left main stem (LMS) to pulmonary artery (PA) fistula as well as a left anterior descending (LAD) coronary artery to PA fistula (figure 2).
Given the degree of ischaemia and coronary anomalies suspected, the patient did undergo coronary angiography, which demonstrated no angiographic evidence of obstructive coronary artery disease, and confirmed the presence of LMS to PA fistula, as well as a LAD coronary artery to PA fistula (figure 2).
Discussion
Coronary artery fistulae may be congenital or acquired – either surgically or iatrogenically. Coronary artery fistulas are present in 0.002% of the general population and visualised in nearly 0.25% of the patients undergoing cardiac catheterisation.1 They can arise from any of the three coronary arteries, most commonly from the right coronary artery (RCA), or LAD coronary artery with the circumflex (LCx) artery rarely involved.
Furthermore, only 5% of coronary artery fistulas are bilateral, and terminate approximately three times more frequently into the PA than single coronary artery fistulas. This is postulated to be secondary to the embryological development of the heart.2 The combination of a LMS and LAD coronary fistula to PA, as presented in this case, has not been previously reported in the literature.
The clinical manifestations of coronary artery fistulae vary considerably and the long-term outcome is poorly understood. Treatment is advocated for symptomatic patients and for those asymptomatic patients who are at risk for future complications.3 Possible therapeutic options include surgical correction and transcatheter embolisation.4
However, what makes this case unique is that, hypertrophic cardiomyopathy (HCM) was additionally diagnosed. As HCM progresses, the degree of myocardial fibrosis may also increase as a result of microvascular ischaemia, left ventricular outflow tract occlusion, and the genotype.5 The CMR findings in this case demonstrated advanced HCM with severe microvascular ischaemia and extensive replacement fibrosis in the maximally hypertrophied segments.
Given the findings of both anomalous coronaries and severe HCM, it was important to determine whether the myocardial ischaemia demonstrated was as a result of the LMS–PA or LAD–PA fistula (a phenomenon called ‘coronary steal syndrome’) and exacerbating the underlying HCM pathogenesis.6 However, there was no angiographic evidence to suggest coronary steal syndrome and the perfusion abnormalities visualised on CMR were attributed to HCM.
As a result of the above investigations, the patient has begun medical management of his HCM, with no interventions deemed necessary for the coronary artery fistulae.
Conflict of interest
None declared.
References
1. Dodge-Khatami A, Mavroudis C, Baker CL. Congenital Heart Surgery Nomenclature and Database Project: anomalies of the coronary arteries. Ann Thorac Surg 2000;69:270–97. https://doi.org/10.1016/S0003-4975(99)01248-5
2. Baim DS, Kline H, Silverman JF. Bilateral coronary artery–pulmonary artery fistulas. Report of five cases and review of the literature. Circulation 1982;65:810–15. https://doi.org/10.1161/01.CIR.65.4.810
3. Warnes CA, Williams RG, Bashore TM et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: executive summary. Circulation 2008;118:2395–451. https://doi.org/10.1161/CIRCULATIONAHA.108.190811
4. Tirilomis T, Aleksic I, Busch T, Zenker D, Ruschewski W, Dalichau H. Congenital coronary artery fistulas in adults: surgical treatment and outcome. Int J Cardiol 2005;98:57–9. https://doi.org/10.1016/j.ijcard.2002.05.001
5. Quarta G, Grasso A, Pasquale F et al. Evolution and clinical importance of fibrosis in HCM. JACC Cardiovasc Imaging 2011;4:1221–3. https://doi.org/10.1016/j.jcmg.2011.04.023
6. Vijayvergiya R, Bhadauria PS, Jeevan H, Mittal BR, Grover A. Myocardial ischemia secondary to dual coronary artery fistulas draining into main pulmonary artery. Int J Cardiol 2010;140:e30–e33. https://doi.org/10.1016/j.ijcard.2008
