This review aims to summarise the cardiovascular complications from cancer treatments and the methods used to prevent, identify, and treat them.
While the field of cardio-oncology is relatively new, it is developing rapidly in the UK. There is a need to develop services to care for the patients with current cardiac problems, to undertake research and education to identify those patients at higher risk of complications, and to apply modern imaging methods and biomarkers to detect problems early and implement prevention strategies. An evidence-based approach is required to enhance delivery of care and prevent cardiovascular toxicity in this patient population.
Introduction
 Advances in cancer care have nearly doubled survival rates in the past 40 years.1 Predictions from European centres2 indicate that there will potentially be a 7.7% and 3.3% drop in age-adjusted cancer mortality in men and women, respectively, during 2016. Much of this survival is in those with childhood and middle-aged cancers who are expected to survive into old age.
Advances in cancer care have nearly doubled survival rates in the past 40 years.1 Predictions from European centres2 indicate that there will potentially be a 7.7% and 3.3% drop in age-adjusted cancer mortality in men and women, respectively, during 2016. Much of this survival is in those with childhood and middle-aged cancers who are expected to survive into old age.
With the increasing life-expectancy of cancer survivors and the general population, the long-term consequences of cancer therapy are becoming evident. Anthracycline-induced heart failure, the classic example of an adverse cardiovascular effect of cancer therapy, was identified in the 1970s.3 Many additional agents are now recognised to have negative impacts on the cardiovascular system.4 These detrimental effects are not limited to heart failure but include ischaemic heart disease, hypertension, electrophysiological disturbances and valvular and pericardial disease, which have all been linked to cancer therapy. These processes can occur in isolation, but are compounded in the presence of traditional cardiovascular risk factors.5
This has led to the recent development of cardio-oncology as a recognised sub-specialty dedicated to the prevention, identification and treatment of cardiovascular complications in patients receiving cancer therapy and those who live as long-term survivors.
This review aims to summarise the cardiovascular complications associated with cancer treatments and the methods used to prevent, identify and treat them in oncology patients.
Heart failure and cancer therapy
Cancer therapy may increase the lifetime risk of heart failure up to 15 times6 and is a prominent cause of iatrogenic heart failure. Highly toxic agents are administered systemically that directly impact on the heart. This effect is not limited to older chemotherapy agents, but also occurs with newer targeted therapies.
Anthracyclines
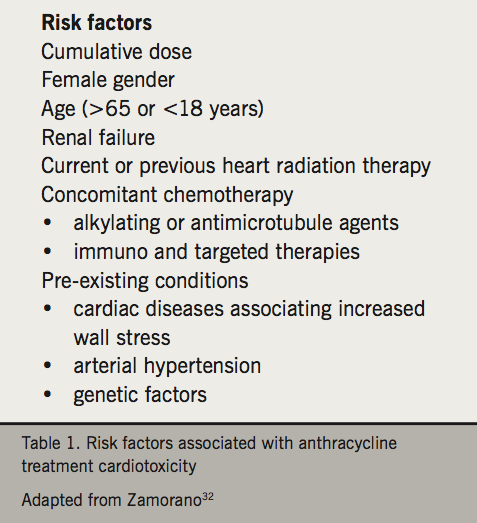
Anthracycline cardiotoxicity was identified in the 1970s,3 and this drug class remains widely used in the treatment for both solid tumours and haematological lymphomas and leukaemias.7 Toxicity is dose-dependent and frequency depends upon the definition and duration of follow up. Historical studies reported a 3–5% incidence of decompensated congestive heart failure for doses up to 400 mg/m2 and 18–48% up to 700 mg/m2 of doxorubicin or equivalent.8 More recent studies report much higher rates at lower doses when reporting clinical heart failure or significant LVEF reductions e.g. 16.2% rate of CCF or LVEF reduction at doxorubicin dose of 300 mg/m2,9 and a large breast cancer study reported ~18% rate of LVEF fall of >10% and ~7% LVEF reduction of >20% at a cumulative doxorubicin dose of 240 mg/m2.10 Significantly higher rates of cardiotoxicity when asymptomatic left ventricular dysfunction or cardiac troponin elevation is reported. It is becoming increasingly evident that baseline patient demographics including age, gender, race, classical cardiovascular risk factors, prior cardiac history and previous cardiotoxic oncology treatments, will influence the absolute risk of anthracycline-induced cardiotoxicity (table 1). The role of genetic susceptibility is also relevant as some apparently low-risk individuals receiving modest anthracycline doses develop significant left ventricular impairment.
The mechanisms of anthracycline cardiotoxicity are multi-factorial.7,11 Anthracyclines induce cardiomyocyte mitochondria to generate free radicals12 that cause structural cellular damage and apoptosis. A recent study has highlighted the role of topoisomerase 2 (Top2)7 in anthracycline mediated cardiotoxicity. Topoisomerases are required for DNA transcription, replication and recombination. Anthracyclines disrupt their normal function leading to DNA strand breaks, mitochondrial dysfunction and more free radicals. Within the human body there are two isoenzymes of Top2, Top2α and Top2β of which the latter is expressed in quiescent myocytes. Animal models of mice13 lacking Top2β are resistant to anthracycline cardiotoxicity.
Dexrazoxane, an iron chelator, reduces the production of free radicals after anthracycline treatment and blocks the interaction of doxorubicin with Top2β. There have been conflicting reports about its efficacy and safety, but a recent Cochrane meta-analysis with longer-term follow-up confirmed that dexrazoxane reduced cardiac complications of anthracycline chemotherapy without increasing the risk of second malignancies.14 This has lead to a recent change in the EMEA license regarding the use of dexrazoxane in paediatric oncology patients.
Biological immunotherapies
Trastuzumab
Over-expression of human epidermal growth factor receptor 2 (HER2) occurs in 30% of breast cancers and indicates a poorer prognosis.15 Inhibition of HER2 with trastuzumab reduces the risk of HER2+ breast cancer recurrence by 9.5% and mortality by 3%. Trastuzumab is associated with an increased risk of left ventricular (LV) systolic dysfunction.16 The absolute risk, analogous to anthracycline cardiotoxicity, discussed above, depends on many patient-related factors including age, cardiovascular risk factors, prior cardiac history and previous cardiotoxic oncology treatments. Co-administration with anthracyclines resulted in an unacceptably high risk of heart failure in the initial metastatic breast cancer trials. Delaying trastuzumab until after anthracyclines has reduced the risk, but in real-world cohorts the risks of trastuzumab-induced LV dysfunction remains clinically relevant (5–15%). The mechanism of LV dysfunction is through the ligand-receptor neuregulin (NRG1) that communicates with HER2.17 Within the normal heart NRG1 mediates: anti-apoptosis, glucose uptake, hypertrophy, sarcomere organisation and calcium cycling. NRG1 knockout mice exhibit absent ventricular trabeculation, myocyte differentiation and develop dilated cardiomyopathy in early life.18
Several guidelines are available to monitor LV function and manage LV dysfunction associated with trastuzumab.19,20 This risk must be balanced against the risk of increased cancer recurrence without HER2 blocking therapy.21 Concomitant use of beta blockers during trastuzumab treatment has been observed to lower the risk of new heart failure,22 but this has yet to be confirmed in interventional trials.
The discovery that HER2 blockade can result in reduced LV function has led to a greater understanding of the importance of NRG1–HER2 signalling in normal and diseased hearts. The NRG1–HER2 pathway is now a promising target for heart failure therapy with infusions of NRG1 in humans sustaining improvement in LV ejection fraction (LVEF).23
The new era of immunotherapy treatments which activate the immune response against the tumour may also pose possible risks to the heart. Although being used across many tumour types (or subgroups of subgroups), particularly of concern as regards potential short- and long-term side effects, are young melanoma patients and the less cardiovascular-fit, lung cancer population. During the last few years several cases of fatal arrhythmias and/or heart failure have been documented in melanoma patients treated with checkpoint inhibitors, particularly in combnination therapy of ipilimumab and nivolumab. These immunotherapies are at the forefront of cancer immunotherapy.24
The robust anticancer activity of these agents has been accompanied by the recognition of new adverse effects, often due to the over-activation of the immune system, that may limit their therapeutic benefit and adversely impact outcomes. Combination treatments in particular, such as approaches using two targeted immunotherapy agents, have higher risk of adverse effects.25
Tyrosine kinase inhibitors (TKIs)
Over-expression of tyrosine kinases is a common feature in numerous cancers and, therefore, represents an attractive therapeutic target. However, a meta-analysis26 indicates that TKIs confer a three-fold risk of heart failure when compared with placebo.
TKIs are proteins that when activated lead to phosphorylation of key substrates within a cell.27 When mutated they cause cell proliferation, angiogenesis and resistance to apoptosis. TKIs are designed to inhibit this but are non-selective between malignant and normal cells. Two main classes of TKIs have been licensed – BCR-ABL-targeted TKIs for chronic myeloid leukaemia (CML) and haematological malignancies, and vascular endothelial growth factor (VEGF)-based TKIs targeting solid tumours including metastatic renal cell carcinoma, sarcoma, gastrointestinal stromal tumor (GIST), hepatocellular carcinoma and thyroid malignancies.
Imatinib, used in the treatment of CML, has been associated with heart failure,28 although this is rare (<1%). Imatinib induces apoptosis by blocking the BCR-ABL tyrosine kinase and disrupting the endoplasmic reticulum leading to an accumulation of misfolded proteins. In an animal model,29 myocyte ABL blockade appeared to be protective of cardiac dysfunction when imatinib was administered. It is possible to design drugs to avoid off-target effects but there is always a risk that this could reduce their efficacy in cancer treatment.
Not all TKIs have been shown to cause cardio-toxicity,27 however, long-term experience with these drugs is limited and data on cardiac dysfunction are frequently collected retrospectively.
Myocardial ischaemia and ischaemic heart disease
Myocardial ischaemia can be induced with chemotherapy by various mechanisms including direct endothelial injury, vasospasm and adverse coagulant profile leading to increased risk of thrombosis.
The fluoropyrimidines, 5-fluorouracil and oral capecitabine, are well-known to induce a syndrome of chest pain and more rarely myocardial ischaemia and LV dysfunction.30 Symptoms occur in up to 35% of patients, the most common being that of chest pain (0–18.6%), and a mortality rate of 0–8% has been reported. Cisplatin is also associated with an increased risk of ischaemic heart disease events with testicular cancer survivors treated with cisplatin having an absolute risk of 8% at 20 years.31
Patients’ cardiovascular risk factors should be managed using the same primary and secondary prevention evidence-based guidelines as the general population. Platelet inhibition can, however, carry an increased risk in oncology patients because of thrombocytopenia, surgery and direct bleeding from tumours.32 Decisions on pharmacological and interventional cardiovascular therapies in this situation can be challenging and should be discussed in a multi-disciplinary team meeting.
Hypertension
Anti-VEGF TKIs and antibodies, e.g. bevacizumab, cause hypertension in up to 50% of patients.33 The presence of pre-existing hypertension is an important risk factor, but drug-induced hypertension can occur in patients without prior hypertension or renal dysfunction. The choice of drug treatment should be based on knowledge of the mechanism(s) of action of these drugs, their risk of causing LV impairment and other cardiac comorbidities. Angiotensin-converting enzyme (ACE) inhibitors are often first choice as they can reduce the risk of LV dysfunction, as well as reducing the blood pressure, and have been shown to be associated with improved cancer prognosis.34 Diltiazem and verapamil should be avoided with anti-VEGF inhibitors as there is an increase in their plasma levels.32
Electrophysiological disturbances
Arrhythmias may be the most common cardiovascular consequence of cancer therapy.35 Many reported in clinical trials are benign but cancer treatments have been implicated in QT prolongation, torsades de pointes, ventricular tachycardia/fibrillation and sudden cardiac death.9
QT prolongation
Oncology patients are at increased risk of QT prolongation.4 Irrespective of chemotherapy prescription, cancer patients are prone to nausea, vomiting and diarrhoea that can cause unfavourable electrolyte imbalances. Concomitant prescription of anti-emetics, antihistamines, antidepressants and antibiotics (particularly macrolides and some antifungal drugs) is not uncommon in cancer patients and contributes to the increased risk.
Arsenic trioxide, used as a second-line agent in acute promyelocytic leukaemia,35 leukaemias and myeloma, is exceptionally potent at prolonging the QT. Between 26% and 93% of patients have measurable increases in QTc, with 30% of patients suffering from a life-threatening arrhythmia.
Vandetanib and lapatinib (TKIs) also prolong the QT interval with 4.3–8% and 6.1% of patients, respectively, extending their QTc >500 ms. Torsades de pointes is described in these groups, however, the exact incidence is unknown.
These data highlight the requirement for baseline measurement and monitoring of QTc in patients undergoing cancer treatment. During periods of acute illness where other risk factors for QT prolongation may become more prominent, attention needs to be focused on correcting the correctable, which could mean temporarily halting the cancer treatment. QTc >500 ms (or change >60 ms) is the threshold to start rationalisation of medication as above this value the risk of arrhythmia becomes prohibitive.32
Atrial fibrillation
Decisions to anticoagulate patients with cancer and atrial fibrillation can be challenging. Traditional risk scores (CHA2DS2-VASC and HAS-BLED), are a useful guide but have not been validated in cancer patients.
Cancer is a prothrombotic state and there is much experience in anticoagulating this group of patients. Typically low molecular weight heparin (LMWH) is preferred, as the international normalised ratio (INR) can be labile. Novel anticoagulants have yet to be fully studied, however, subgroup analysis36 suggests a possible favourable profile compared with warfarin and heparins, although this requires confirmation. However, cancer patients may also have increased bleeding risks and thrombocytopenia may be an issue, presenting the same challenge as in ischaemic heart disease.
Radiotherapy and cardiovascular disease
Radiotherapy has been linked to increased cardiovascular risk (figure 1a–c). While the myocardium was initially thought to be relatively insensitive to the effects of radiotherapy, the vascular endothelium is particularly radiation sensitive,37 with capillaries and small arteries being the most susceptible to damage. Larger arteries are less vulnerable, but when damage does occur it can potentially be fatal.
Patients with lymphoma, head and neck cancers or breast cancer, often receive radiotherapy to the thorax. Left-sided breast cancer radiotherapy can affect the left main and anterior descending coronaries while radiotherapy for Hodgkin lymphomas can also affect the right and circumflex arteries.38
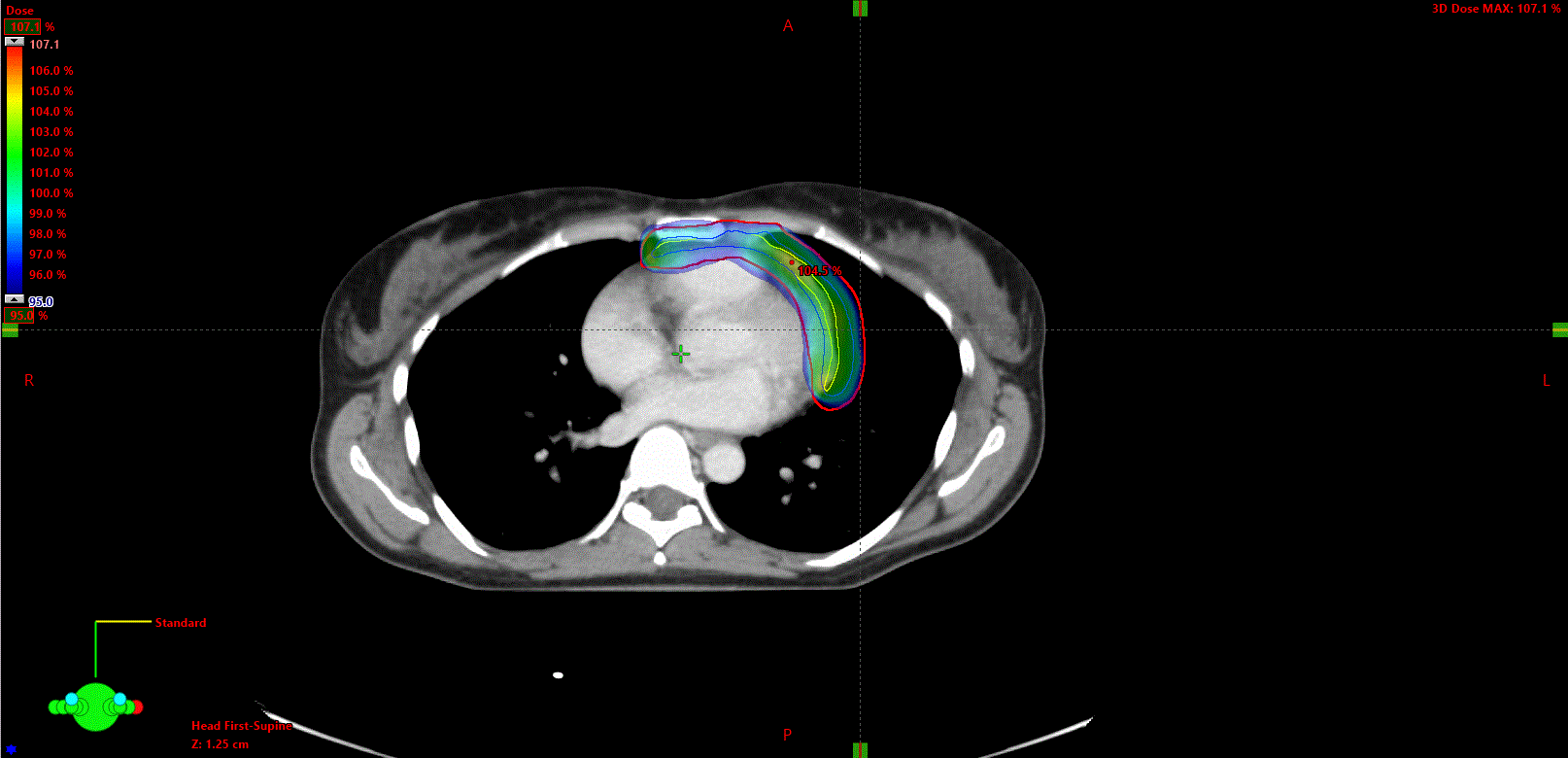
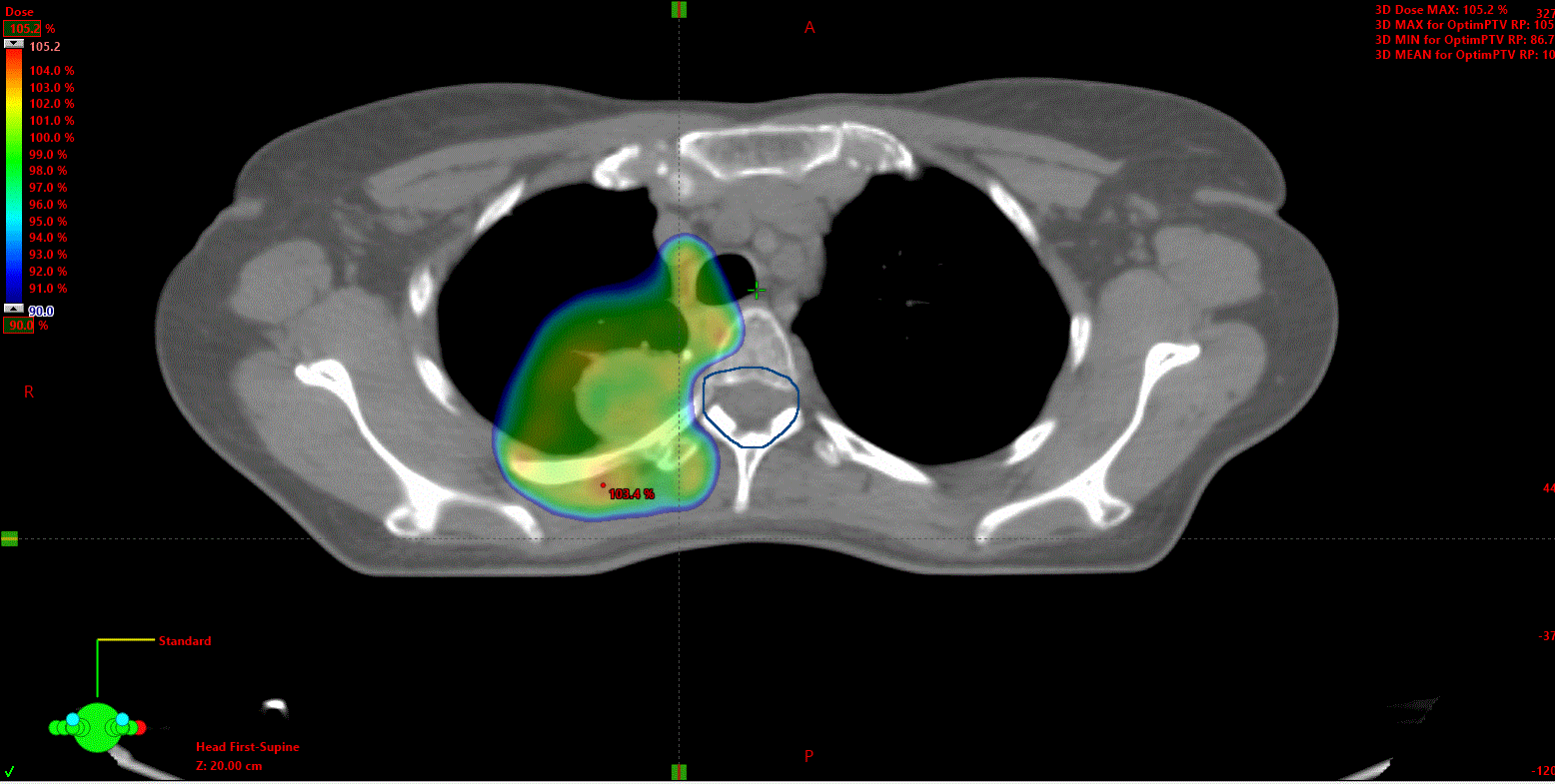
Long-term follow-up of breast cancer survivors treated in the 1970s showed radiotherapy to be associated with a hazard ratio of 2.55 (95% confidence interval [CI] 1.55–4.19) for myocardial infarction and 1.72 (95% CI 1.22–2.41) for congestive heart failure.39
There are, however, other studies of breast cancer survivors40-42 that have reported no excess cardiovascular mortality when comparing those who received left- versus right-sided radiotherapy. They suggest that improved radiotherapy techniques since the 1980s reduce the risk to the heart. A more contemporary study suggests the risk of radiation-induced myocardial infarction is dose-dependent, with a 7.4% relative risk increase per Grey of radiation to the heart. The clinical consequences, therefore, depend on the baseline absolute risk. In women with pre-existing vascular disease or risk factors, event rates are higher and seen earlier after treatment (within 10 years). Conversely, for lower-risk patients the delay between radiotherapy and manifestation of cardiovascular disease is over 10 years. A recent study also identified left-sided radiotherapy for breast cancer as a risk factor for developing heart failure with preserved ejection fraction (HFpEF). This may be related to accelerated endothelial dysfunction and direct myocardial toxicity with fibrosis.43
Radiotherapy for head and neck cancer confers a greatly increased risk of stroke.44,45 At 15 years the absolute risk of ischaemic stroke is 12%. Angiographic comparison46 of these lesions compared with non-radiotherapeutic field lesions indicates that they are typically longer and taper to maximum stenotic point at the end of the lesion.
Management of this vascular damage can be difficult. In vitro study of human endothelium indicates that statins improve the inflammatory and thrombotic profile and would support trials of their use in vivo.47 Comparing percutaneous intervention versus endarterectomy for traditional carotid atherosclerotic lesions indicates non-inferiority of percutaneous intervention;48 this combined with the knowledge that previously irradiated areas are surgically challenging due to fibrotic changes further supports percutaneous approaches in this population.
Surveillance strategies in cardio-oncology
There is a lack of universal agreement on the most appropriate prospective cardiovascular screening strategy in oncology patients, especially when they are receiving newer agents.49,50 Several imaging modalities are used, with echocardiography the most commonly used for surveillance in patients receiving treatments putting them at risk of LV dysfunction.
Echocardiography
The most common measure of LV function is the ejection fraction (LVEF). Consistency on the method used to determine LVEF is crucial and Simpson’s biplane method is the recommended technique for 2D imaging. 2D imaging techniques however are only capable of detecting changes in LVEF greater than ~10%,51 therefore, smaller but significant deteriorations in LVEF may not be detected using this method. A decrease in LVEF of >10% to a value <53% has been proposed as a definition of cancer therapy-related cardiac dysfunction.52 Various protocols are suggested depending on the resources available at each individual institution.
Indices of ventricular strain, such as global longitudinal peak systolic strain (GLS), appear to be more sensitive and specific predictors of early cardiotoxicity than simple measurement of LVEF. An 11% decrease in GLS, measured using speckled tracking echocardiography (STE), has a sensitivity of 65% and specificity of 94% for later cardiotoxicity.53 GLS combined with traditional biplane methods of determining LVEF has a beneficial net reclassification index (0.77, p=0.036). This places 48% of patients, previously categorised as low risk but who in practice suffered cardiac events, into the higher risk category while subsequently downgrading 29% of patients classified as high risk into the low risk category.
Echocardiography also offers evaluation of all elements of cardiac function, including diastolic function, right ventricular function, valvular function, atrial dimensions, pulmonary artery pressure and pericardial disease.
Limitations of echocardiography can include reproducibility, poor acoustic windows in up to a third of patients, particularly if recent left breast or chest wall surgery; obesity or chronic lung disease. Discomfort for oncology patients post-surgery, left chest radiotherapy with skin complications and rib metastases are further difficulties for echocardiography in this patient cohort.
Echocardiography is relatively cheap and widely available in UK hospitals. The protocols can be adapted for surveillance in oncology patients, for example, undertaking a detailed baseline echo according to British Society of Echocardiography (BSE) guidelines with follow-up scans focused on aspects of specific concern, e.g. LV function, thereby shortening the scan time and increasing both comfort for the patient and flow of patients through the service.
Cardiac magnetic resonance (CMR)
Cardiac magnetic resonance (CMR) imaging has been shown to be superior to echocardiography in assessing changes in ventricular dimensions and function.54 It is highly reproducible, an essential requirement in the follow-up of this specific patient group. There is also the benefit of not subjecting patients to further ionising radiation when compared with nuclear techniques.
Beyond determining dimensions, CMR can detect the tissue changes of oedema, inflammation, scarring and fibrosis, all of which can occur in relation to cancer therapy.55 Currently, cost, scan duration and availability prevent its widespread use in these patients.
Nuclear imaging
The use of radionuclide angiocardiography has been established in many oncology centres for the serial assessment of LVEF.56 There is a high degree of reliability and reproducibility when compared with echocardiography,57 but exposure to additional radiation is a concern, especially when non-irradiating alternatives are available. Where high quality reproducible echocardiography is not available, multigated acquisition (MUGA) may still be considered, but echocardiography has clear advantages.
High-sensitivity troponin (hsTrop)
hsTrop elevation from baseline in those treated with chemotherapy has been shown to identify patients at increased risk of cardiotoxicity. Detection of increased hsTrop I post-trastuzumab cycle has a hazard ratio 22.9 for heart failure.19 When combined with GLS, the overall sensitivity is increased to 93% and a negative predictive value of 91% achieved. It is likely that evidence-based guidelines for the serial measurement of hsTrop will be developed. Furthermore, the ease of obtaining hsTrop values from a standard blood sample, its universal availability and low cost of the assay combined with its high reproducibility suggest that systematic use of hsTrop may well prove to be cost-effective.

Progress and training
There has been a rapid growth in the field of cardio-oncology in response to the growing efficacy and long-term survivorship achieved by modern oncology. In response, the International Cardio-Oncology Society (http://icosna.org) and Canadian Cardio-Oncology Network (http://cardiooncology.ca) have been formed and, in the UK, the British Cardio-Oncology Society was created in 2014 (http://bc-os.org). The Global Cardio-Oncology Summit was hosted in London in September 2017 (see www.bc-os.org). The new Cardio-Oncology Journal is into its second volume.
A cardio-oncology training programme, based on the US training model, has been proposed,58 including two weeks’ exposure for medical students, one month for residents and a year for cardiovascular and oncology registrars. The recent position paper from the European Society of Cardiology (ESC)30 provides information and opinion regarding the multiple facets of managing cardiovascular problems in oncology patients, while acknowledging that it is not a formal clinical practice guideline. Within the UK, dedicated services are currently offered at the Royal Brompton Hospital, Freeman Hospital in Newcastle, Liverpool Heart and Chest Hospital, UCLH and other centres (table 2). A recent survey,58 indicates that two-thirds of surveyed hospitals in the US are adding cardio-oncology services to their roster.
Conclusion
The field of cardio-oncology is relatively new but developing rapidly in the UK. There is a need to develop services to care for the patients with current cardiac problems, to undertake research and education to identify those patients at higher risk of complications, and to apply modern imaging methods and biomarkers to detect problems early and implement prevention strategies. An evidence-based approach is required to enhance delivery of care and prevent cardiovascular toxicity in this patient population.
Conflict of interest
JB: no conflict. AL: none declared. CP has received honoraria for speaking at educational meetings or advisory boards from Amgen, Ferring, Novartis, Pfizer and Roche. SR is on the advisory board for Clinigen, the manufacturer of Cardioxane. K-KS: no conflict.
Key messages
- The growing pool of survivors will drive the requirement for ongoing research and service provision
- Further research is required to provide an evidence base for effective preventive and therapeutic strategies
- Serial echocardiography using global longitudinal peak systolic strain is the current monitoring modality of choice for detecting subtle deterioration in left ventricular ejection fraction
- Decisions balancing cancer therapy against cardiovascular deterioration are complex requiring an integrated multi-disciplinary approach
References
1. Cancer Research UK. Cancer Survival Statistics. Available at: http://www.cancerresearchuk.org/health-professional/cancer-statistics/survival#heading-Zero [accessed 25 August 2016].
2. Malvezzi M, Carioli G, Bertuccio P et al. European cancer mortality predictions for the year 2016 with focus on leukaemias. Ann Oncol 2016;27:725–31. https://doi.org/10.1093/annonc/mdw022
3. Lefrak EA, Pitha J, Rosenheim S, Gottlieb JA. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer 1973;32:302–14. https://doi.org/10.1002/1097-0142(197308)32:2<302::AID-CNCR2820320205>3.0.CO;2-2
4. Yeh ETH, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol 2009;53:2231–47. https://doi.org/10.1016/j.jacc.2009.02.050
5. Armstrong GT, Oeffinger KC, Chen Y et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol 2013;31:3673–80. https://doi.org/10.1200/JCO.2013.49.3205
6. Oeffinger KC, Mertens AC, Sklar CA et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 2006;355:1572–82. https://doi.org/10.1056/NEJMsa060185
7. Vejpongsa P, Yeh ETH. Prevention of anthracycline-induced cardiotoxicity: challenges and opportunities. J Am Coll Cardiol 2014;64:938–45. https://doi.org/10.1016/j.jacc.2014.06.1167
8. Wouters KA, Kremer LCM, Miller TL, Herman EH, Lipshultz SE. Protecting against anthracycline-induced myocardial damage: a review of the most promising strategies. Br J Haematol 2005;131:561–78. https://doi.org/10.1111/j.1365-2141.2005.05759.x
9. Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials Cancer 2003;97:2869-79 https://doi.org/10.1002/cncr.11407
10. Perez EA, Suman VJ, Davidson NE et al. Effect of doxorubicin plus cyclophosphamide on left ventricular ejection fraction in patients with breast cancer in the North Central Cancer Treatment Group N9831 Intergroup Adjuvant Trial. J Clin Oncol. 2004 Sep 15;22(18):3700-4 https://doi.org/10.1200/JCO.2004.03.516
11. Sawyer DB, Peng X, Chen B, Pentassuglia L, Lim CC. Mechanisms of anthracycline cardiac injury: can we identify strategies for cardioprotection? Prog Cardiovasc Dis 2010;53:105–13. https://doi.org/10.1016/j.pcad.2010.06.007
12. Horenstein MS, Vander Heide RS, L’Ecuyer TJ. Molecular basis of anthracycline-induced cardiotoxicity and its prevention. Mol Genet Metab 2000;71:436–44. https://doi.org/10.1006/mgme.2000.3043
13. Lipshultz SE, Lipsitz SR, Kutok JL et al. Impact of hemochromatosis gene mutations on cardiac status in doxorubicin-treated survivors of childhood high-risk leukemia. Cancer 2013;119:3555–62. https://doi.org/10.1002/cncr.28256
14. Vejpongsa P, Yeh ETH. Topoisomerase 2β: a promising molecular target for primary prevention of anthracycline-induced cardiotoxicity. Clin Pharmacol Ther 2013;95:45–52. https://doi.org/10.1038/clpt.2013.201
15. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235:177–82. https://doi.org/10.1126/science.3798106
16. Moja L, Tagliabue L, Balduzzi S et al. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev 2012;(4):CD006243. https://doi.org/10.1002/14651858.CD006243.pub2
17. Yin HK, Li XY, Jiang ZG, Zhou MD. Progress in neuregulin/ErbB signaling and chronic heart failure. World J Hypertens 2015;5:63–73. https://doi.org/10.5494/wjh.v5.i2.63
18. Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature 1995;378:386–90. https://doi.org/10.1038/378386a0
19. Curigliano G, Cardinale D, Suter T et al. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann Oncol 2012;23(suppl 7):vii155–vii166. https://doi.org/10.1093/annonc/mds293
20. Jones AL, Barlow M, Barrett-Lee PJ et al. Management of cardiac health in trastuzumab-treated patients with breast cancer: updated United Kingdom National Cancer Research Institute recommendations for monitoring. Br J Cancer 2009;100:684–92. https://doi.org/10.1038/sj.bjc.6604909
21. Yu AF, Yadav NU, Lung BY et al. Trastuzumab interruption and treatment-induced cardiotoxicity in early HER2-positive breast cancer. Breast Cancer Res Treat 2015;149:489–95. https://doi.org/10.1007/s10549-014-3253-7
22. Seicean S, Seicean A, Alan N, Plana JC, Budd GT, Marwick TH. Cardioprotective effect of beta-adrenoceptor blockade in patients with breast cancer undergoing chemotherapy: follow-up study of heart failure. Circ Heart Fail 2013;6:420–6. https://doi.org/10.1161/CIRCHEARTFAILURE.112.000055
23. Jabbour A, Hayward CS, Keogh AM et al. Parenteral administration of recombinant human neuregulin-1 to patients with stable chronic heart failure produces favourable acute and chronic haemodynamic responses. Eur J Heart Fail 2011;13:83–92. https://doi.org/10.1093/eurjhf/hfq152
24. Varricchi G, Marone G, Mercurio V et al. Immune checkpoint inhibitors and cardiac toxicity: an emerging issue. Curr Med Chem 2017:online first. https://doi.org/10.2174/0929867324666170407125017
25. Jain V, Bahia J, Mohebtash M et al. Cardiovascular complications associated with novel cancer immunotherapies. Curr Treat Options Cardiovasc Med 2017;19:36. https://doi.org/10.1007/s11936-017-0532-8
26. Ghatalia P, Morgan CJ, Je Y et al. Congestive heart failure with vascular endothelial growth factor receptor tyrosine kinase inhibitors. Crit Rev Oncol Hematol 2017;94:228–37. https://doi.org/10.1016/j.critrevonc.2014.12.008
27. Orphanos GS, Ioannidis GN, Ardavanis AG. Cardiotoxicity induced by tyrosine kinase inhibitors. Acta Oncol (Madr) 2009;48:964–70. https://doi.org/10.1080/02841860903229124
28. Chen MH, Kerkela R, Force T. Mechanisms of cardiomyopathy associated with tyrosine kinase inhibitor cancer therapeutics. Circulation 2008;118:84–95. https://doi.org/10.1161/CIRCULATIONAHA.108.776831
29. Fernández A, Sanguino A, Peng Z et al. An anticancer C-Kit kinase inhibitor is reengineered to make it more active and less cardiotoxic. J Clin Invest 2007;117:4044–54. https://doi.org/10.1172/JCI32373
30. Polk A, Vaage-Nilsen M, Vistisen K, Nielsen DL. Cardiotoxicity in cancer patients treated with 5-fluorouracil or capecitabine: a systematic review of incidence, manifestations and predisposing factors. Cancer Treat Rev 2013;39:974–84. https://doi.org/10.1016/j.ctrv.2013.03.005
31. Haugnes HS, Wethal T, Aass N et al. Cardiovascular risk factors and morbidity in long-term survivors of testicular cancer: a 20-year follow-up study. J Clin Oncol 2010;28:4649–57. https://doi.org/10.1200/JCO.2010.29.9362
32. Zamorano JL, Lancellotti P, Rodriguez Mu-oz D et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines. The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J 2016;37:2768–801. https://doi.org/10.1093/eurheartj/ehw211
33. Wu S, Chen JJ, Kudelka A, Lu J, Zhu X. Incidence and risk of hypertension with sorafenib in patients with cancer: a systematic review and meta-analysis. Lancet Oncol 2008;9:117–23. https://doi.org/10.1016/S1470-2045(08)70003-2
34. Hall PS, Harshman LC, Srinivas S, Witteles RM. The frequency and severity of cardiovascular toxicity from targeted therapy in advanced renal cell carcinoma patients. JACC Heart Fail 2013;1:72–8. https://doi.org/10.1016/j.jchf.2012.09.001
35. Tamargo J, Caballero R, Delpon E. Cancer chemotherapy and cardiac arrhythmias: a review. Drug Saf 2015;38:129–52. https://doi.org/10.1007/s40264-014-0258-4
36. Larsen TB, Nielsen PB, Skjoth F, Rasmussen LH, Lip GYH. Non-vitamin K antagonist oral anticoagulants and the treatment of venous thromboembolism in cancer patients: a semi systematic review and meta-analysis of safety and efficacy outcomes. PLoS One 2014;9:e114445. https://doi.org/10.1371/journal.pone.0114445
37. Fajardo LF, Berthrong M. Vascular lesions following radiation. Pathol Annu 1988;23(Pt 1):297–330.
38. Darby SC, Ewertz M, McGale P et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987–98. https://doi.org/10.1056/NEJMoa1209825
39. Hooning MJ, Botma A, Aleman BMP et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst 2007;99:365–75. https://doi.org/10.1093/jnci/djk064
40. Nixon AJ, Manola J, Gelman R et al. No long-term increase in cardiac-related mortality after breast-conserving surgery and radiation therapy using modern techniques. J Clin Oncol 1998;16:1374–9. https://doi.org/10.1200/JCO.1998.16.4.1374
41. Vallis KA, Pintilie M, Chong N et al. Assessment of coronary heart disease morbidity and mortality after radiation therapy for early breast cancer. J Clin Oncol 2002;20:1036–42. https://doi.org/10.1200/JCO.2002.20.4.1036
42. Darby S, McGale P, Peto R, Granath F, Hall P, Ekbom A. Mortality from cardiovascular disease more than 10 years after radiotherapy for breast cancer: nationwide cohort study of 90 000 Swedish women. BMJ 2003;326:256–7. https://doi.org/10.1136/bmj.326.7383.256
43. Saiki H, Petersen IA, Scott CG et al. Risk of heart failure with preserved ejection fraction in older women after contemporary radiotherapy for breast cancer clinical perspective. Circulation 2017;135:1388–96 https://doi.org/10.1161/CIRCULATIONAHA.116.025434
44. Dorresteijn LDA, Kappelle AC, Boogerd W et al. Increased risk of ischemic stroke after radiotherapy on the neck in patients younger than 60 years. J Clin Oncol 2002;20:282–8. https://doi.org/10.1200/JCO.2002.20.1.282
45. Gujral DM, Shah BN, Chahal NS, Senior R, Harrington KJ, Nutting CM. Clinical features of radiation-induced carotid atherosclerosis. Clin Oncol 2014;26:94–102. https://doi.org/10.1016/j.clon.2013.10.002
46. Shichita T, Ogata T, Yasaka M et al. Angiographic characteristics of radiation-induced carotid arterial stenosis. Angiology 2009;60:276–82. https://doi.org/10.1177/0003319709335905
47. Gaugler M-H, Vereycken-Holler V, Squiban C, Vandamme M, Vozenin-Brotons M-C, Benderitter M. Pravastatin limits endothelial activation after irradiation and decreases the resulting inflammatory and thrombotic responses. Radiat Res 2005;163:479–87. https://doi.org/10.1667/RR3302
48. Yadav JS, Wholey MH, Kuntz RE et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med 2004;351:1493–1501. https://doi.org/10.1056/NEJMoa040127
49. Bloom MW, Hamo CE, Cardinale D et al. Cancer therapy-related cardiac dysfunction and heart failure. Part 1: definitions, pathophysiology, risk factors, and imaging. Circ Heart Fail 2016;9:e002661. https://doi.org/10.1161/CIRCHEARTFAILURE.115.002661
50. Hamo CE, Bloom MW, Cardinale D et al. Cancer therapy-related cardiac dysfunction and heart failure. Part 2: prevention, treatment, guidelines, and future directions. Circ Heart Fail 2016;9:e002843. https://doi.org/10.1161/CIRCHEARTFAILURE.115.002843
51. Plana JC, Galderisi M, Barac A et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2014;27:911–39. https://doi.org/10.1016/j.echo.2014.07.012
52. Thavendiranathan P, Grant AD, Negishi T, Plana JC, Popovic ZB, Marwick TH. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: application to patients undergoing cancer chemotherapy. J Am Coll Cardiol 2013;61:77–84. https://doi.org/10.1016/j.jacc.2012.09.035
53. Negishi K, Negishi T, Hare JL, Haluska BA, Plana JC, Marwick TH. Independent and incremental value of deformation indices for prediction of trastuzumab-induced cardiotoxicity. J Am Soc Echocardiogr 2013;26:493–8. https://doi.org/10.1016/j.echo.2013.02.008
54. Grothues F, Smith GC, Moon JCC et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol 2002;90:29–34. https://doi.org/10.1016/S0002-9149(02)02381-0
55. Thavendiranathan P, Wintersperger BJ, Flamm SD, Marwick TH. Cardiac MRI in the assessment of cardiac injury and toxicity from cancer chemotherapy. Circ Cardiovasc Imaging 2013;6:1080–91. https://doi.org/10.1161/CIRCIMAGING.113.000899
56. Alexander J, Dainiak N, Berger HJ et al. Serial assessment of doxorubicin cardiotoxicity with quantitative radionuclide angiocardiography. N Engl J Med 1979;300:278–83. https://doi.org/10.1056/NEJM197902083000603
57. Schwartz RG, Jain D, Storozynsky E. Traditional and novel methods to assess and prevent chemotherapy-related cardiac dysfunction noninvasively. J Nucl Cardiol 2013;20:443–64. https://doi.org/10.1007/s12350-013-9707-1
58. Lenihan DJ, Hartlage G, DeCara J et al. Cardio-oncology training: a proposal from the International Cardioncology Society and Canadian Cardiac Oncology Network for a new multidisciplinary specialty. J Card Fail 2016;22:465–71. https://doi.org/10.1016/j.cardfail.2016.03.012
