
Introduction
Cardiac resynchronisation therapy (CRT) is a standard treatment for patients with heart failure (HF), impaired left ventricular (LV) function and a wide QRS complex. The therapy emerged following the recognition that disturbances in inter- and intra-ventricular conduction contribute to LV dysfunction. Since its first clinical use,1,2 robust evidence from randomised-controlled trials (RCTs) has supported its use in patients with severe and mild HF. In combination with defibrillation (CRT-D), CRT reduces mortality from pump failure and sudden, arrhythmic cardiac death (SCD).
Since the last RCTs on CRT,3 the field has undergone a period of ‘soul-searching’, dominated by subanalyses of RCTs and observational studies geared towards risk stratification and therapy optimisation. A similar scrutiny has been adopted in the field of implantable cardioverter-defibrillator (ICD) therapy. Although indications for CRT have not changed substantially in the past 10 years, we are now clearer as to which patients benefit the most. Advances in device hardware and lead technology, as well as a more systematic approach to medical therapy have contributed to improving patient outcomes.
CRT-D versus CRT pacemaker (CRT-P)
The cost of CRT-D versus CRT-P has fuelled the debate as to whether CRT-D is superior to CRT-P, particularly as CRT-P also reduces SCD.4 No RCT has addressed this issue. Data from meta-analyses are inconsistent. A network meta-analysis of 13 randomised trials (12,638 patients) showed that CRT-D reduced total mortality by 19% compared with CRT-P.5 On the other hand, meta-analyses undertaken by the National Institute of Health and Care Excellence (NICE) did not show a survival benefit from CRT-D over CRT-P.6 The lack of clear guidance from guideline groups as to the choice of CRT-D versus CRT-P is reflected in the wide variation in device choice. While, in the US, CRT-P accounts for only 14% of all CRT implantations,7 the figure is 30% in Japan and 48% in the UK.8 The use of CRT-P in Europe is increasing.9
Aetiology in the post-DANISH era
The landmark trials of CRT were not designed to explore the effect of HF aetiology on CRT outcomes. Several observational studies have since emerged. In a cohort of 1,122 patients, Kutyifa et al. showed that CRT-D was associated with a reduction (30%) in all-cause mortality compared with CRT-P in ischaemic cardiomyopathy (ICM), but not in non-ischaemic cardiomyopathy (NICM).10 In a European cohort study of 5,307 patients, Barra et al. also found that CRT-D was superior to CRT-P in ICM, but not in NICM.11 In a cohort of 1,500 patients, we found that when stratified according to aetiology, CRT-D was associated with a lower total mortality (38%) than CRT-P in ICM, but not in NICM (figure 1).12
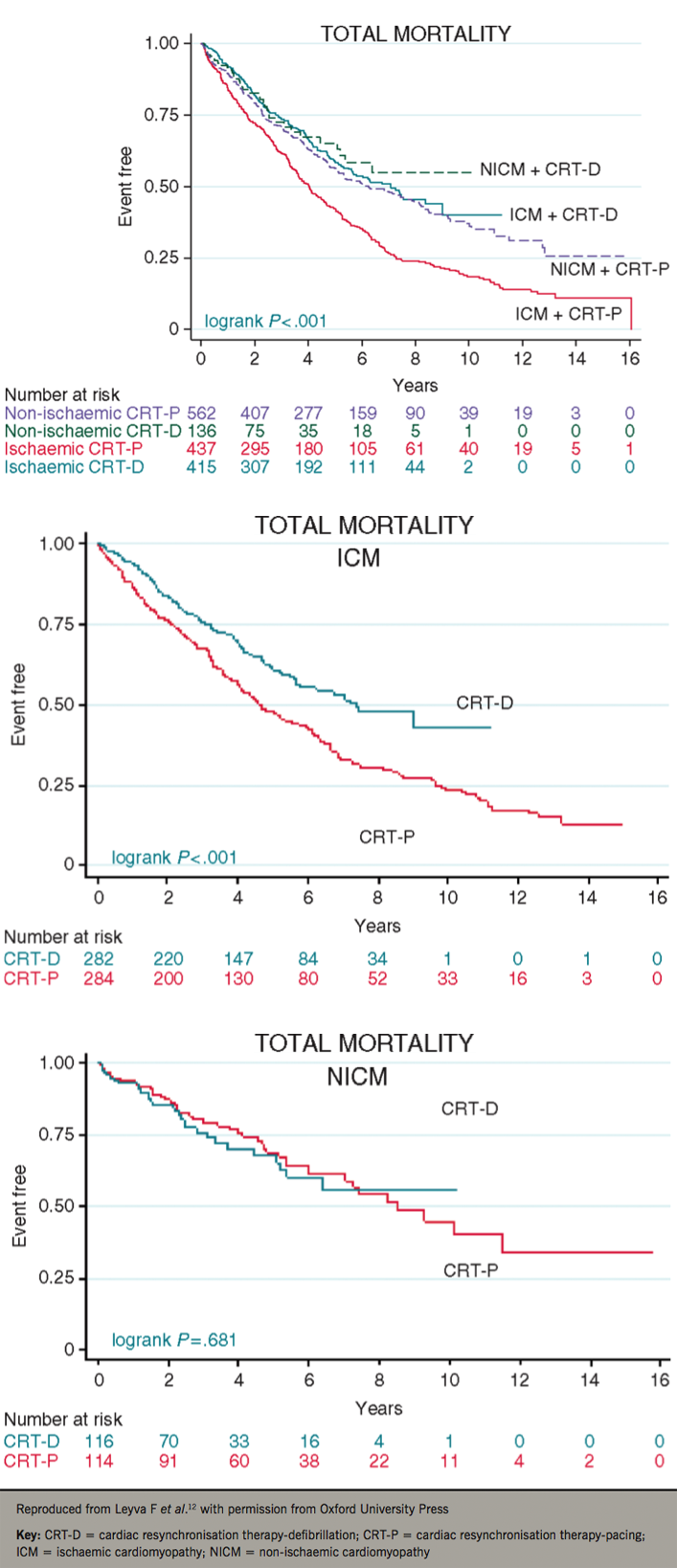
In the DANISH (Danish Study to Assess the Efficacy of ICDs in Patients with Non-ischemic Systolic Heart Failure on Mortality) study, 1,116 patients with NICM were randomised to usual clinical care (control group) or ICD, with or without CRT.13 In the treatment and control groups, 58% received CRT. After a median follow-up period of 67.6 months, there was no difference in total mortality, despite a 50% reduction in SCD. This finding has raised doubts as to the benefit of CRT-D over CRT-P in NICM. Importantly, however, DANISH may be underpowered to show a benefit from ICDs in NICM. In this respect, the NICE network meta-analysis undertaken prior to DANISH required 1,457 patients to show a survival benefit from ICDs in NICM,6 and even higher numbers were used in recent meta-analyses that include DANISH.14 On this basis, DANISH undoubtedly changes our perspective as to the use of CRT-D versus CRT-P in NICM, but it is not the ultimate trial. On the other hand, DANISH confirms the survival advantage of CRT-D in younger patients (<59 years).
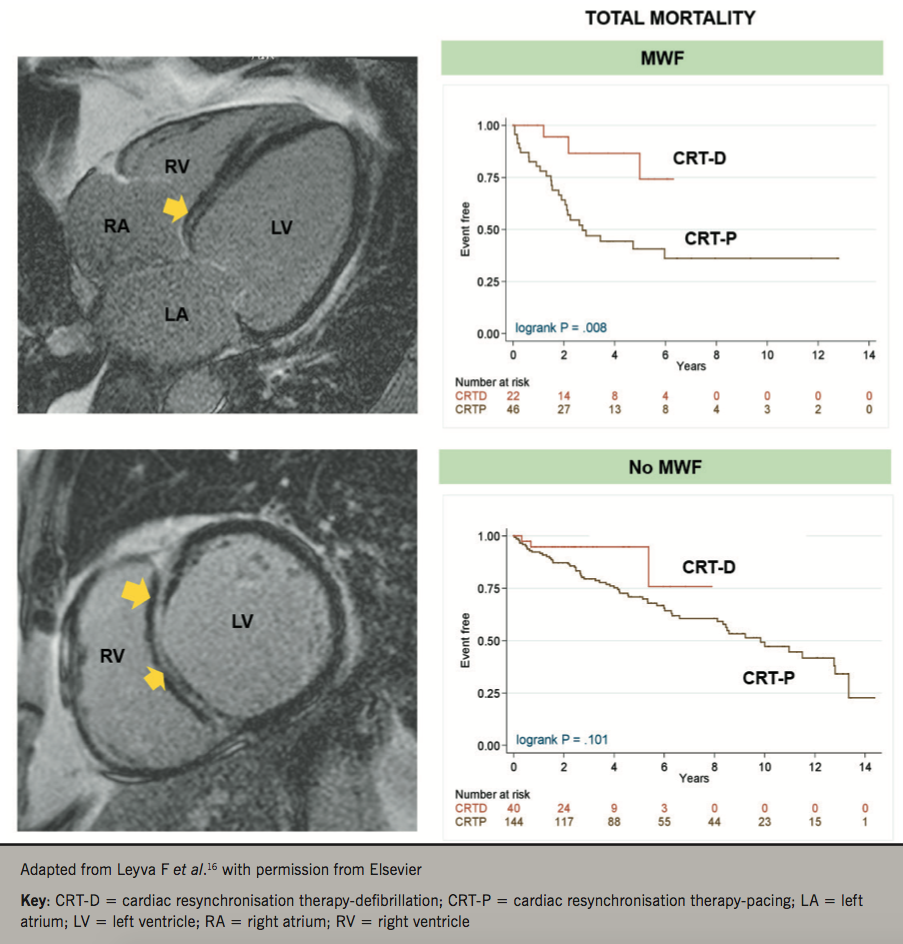
Not all NICMs are the same
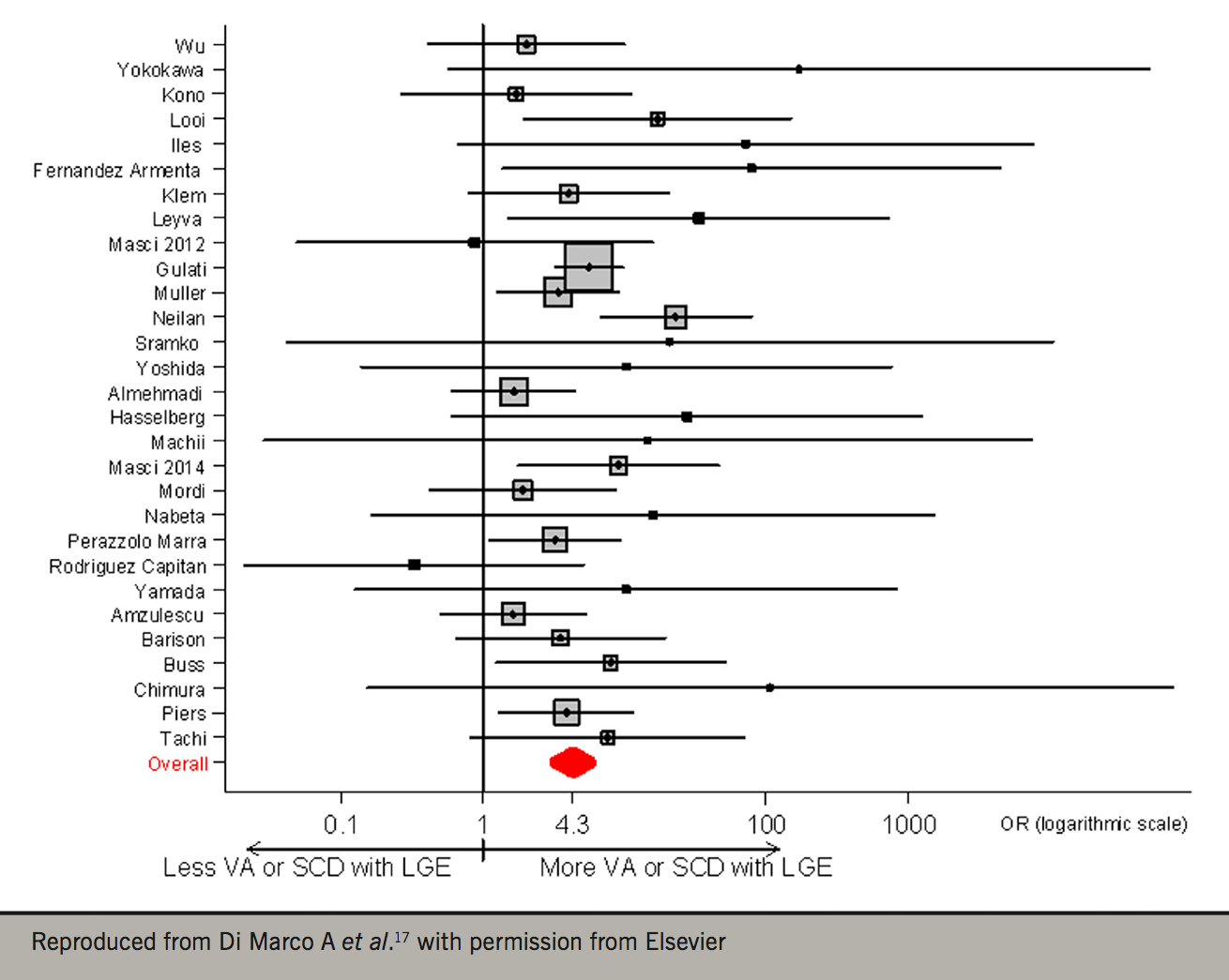
Traditionally, even in CRT RCTs, the diagnosis of NICM has been made on the basis of echocardiography and coronary angiography. We should consider, however, that these imaging modalities do not provide tissue characterisation. Cardiovascular magnetic resonance (CMR) is now the gold-standard for the characterisation of myocardial phenotypes.15 Using CMR, we have recently shown that LV mid-wall fibrosis detected by CMR modulates the outcomes of CRT (figure 2).16 In 252 patients with NICM, LV mid-wall fibrosis predicted total mortality, death from pump failure and SCD. Total mortality was lower (77%) with CRT-D compared with CRT-P in patients with mid-wall fibrosis, but not in those without. These findings are consistent with a recent meta-analysis in which myocardial fibrosis on CMR was independently associated with ventricular arrhythmia and SCD in NICM. On this basis, it appears that assessment of myocardial fibrosis with CMR could be a powerful tool in choosing between CRT-D and CRT-P in patients with NICM (figure 3).17
CRT and right ventricular pacing
In the background that HF is common in the context of right ventricular pacing,18,19 in the recent European CRT Survey II, 23.2% of CRT patients were upgraded from a previous pacemaker or ICD.20 In BLOCK-HF (Biventricular versus Right Ventricular Pacing in Heart Failure Patients with Atrioventricular Block),21 in which patients with HF and conventional indications for pacing were randomised to CRT or right ventricular (RV) pacing, CRT was associated with a 26% reduction in the primary composite end point of total mortality, urgent HF care or an increase in LV end-systolic volume. Although BLOCK-HF did not clarify the cut-off of LV ejection fraction (EF) one should use at the time of upgrading, there is consensus that patients with conventional indication for pacing and impaired LV function, perhaps <50%, should preferentially undergo CRT. In this context, an observational study has shown that CRT-D may be preferable to CRT-P.22
Medical therapy and SCD
The incidence of SCD in the landmark trials of CRT from the early 2000s was high.23,24 This, however, may be changing. In a recent meta-analysis comprising 40,195 patients with HF enrolled in 12 clinical trials from 1995 to 2014, Shen et al. have shown a 44% decline in the rate of SCD across trials.25 Although these authors invoked the possible effects of angiotensin receptor neprolysin inhibitors on SCD, the trials predated their use. Moreover, the apparent decline in SCD in HF could reflect selection trends for HF trials and not necessarily the incidence of SCD in potential CRT recipients, nor indeed, in the general population. Nevertheless, it seems reasonable to consider that SCD in HF may now be less frequent than in the early 2000s.
CRT in atrial fibrillation
Atrial fibrillation (AF) occurs in up to 25% of patients with HF in New York Heart Association (NYHA) class II /III, and in up to 50% of patients in NYHA class IV.26 In AF, CRT cannot correct atrioventricular dyssynchony, but only inter- and intra-ventricular dyssynchrony. In the largest observational study to date, Gasparini et al.27 explored the outcome of CRT in patients with permanent AF, in combination with atrioventricular junction ablation or rate-slowing drugs. Over a median follow-up of 37 months, CRT with ablation had comparable risks of total and cardiac mortality to patients in sinus rhythm, while AF treated with rate-slowing drugs had a worse outcome. Importantly, CRT recipients with AF and atrioventricular junction ablation have a lower risk of appropriate and inappropriate ICD shocks, as well as fewer all-cause and HF hospitalisations.28 Randomised trials of CRT in patients with AF have not been undertaken.
QRS morphology and duration
In contrast to early studies suggesting a benefit from CRT in patients with a QRS <120 ms,29-31 the Echo-CRT study32 showed that CRT in this patient group led to no benefit, compared with ICD. The trial was stopped prematurely for futility after the finding of a higher mortality with CRT-D. In essence, CRT is of no survival benefit in patients with a QRS <120 ms. The threshold of 130 ms for CRT seems sensible.33
Subanalyses of both REVERSE (Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction)34 and MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy)35 suggested a reduced benefit in patients with non-left bundle branch block (LBBB) QRS morphology. However, LBBB morphology is not an independent predictor of total mortality according to a network meta-analysis of CRT trials.36 On balance, it would appear that QRS morphology is only relevant at a QRS <150 ms, as recommended in recent NICE guidelines. In this context, however, the clinician implanting an ICD without an LV lead, as recommended by NICE for some patient groups, invites a future upgrade to a CRT-D system, which carries a high risk of infections and other complications.37 For the clinician and the patient, one should minimise the number of interventions.
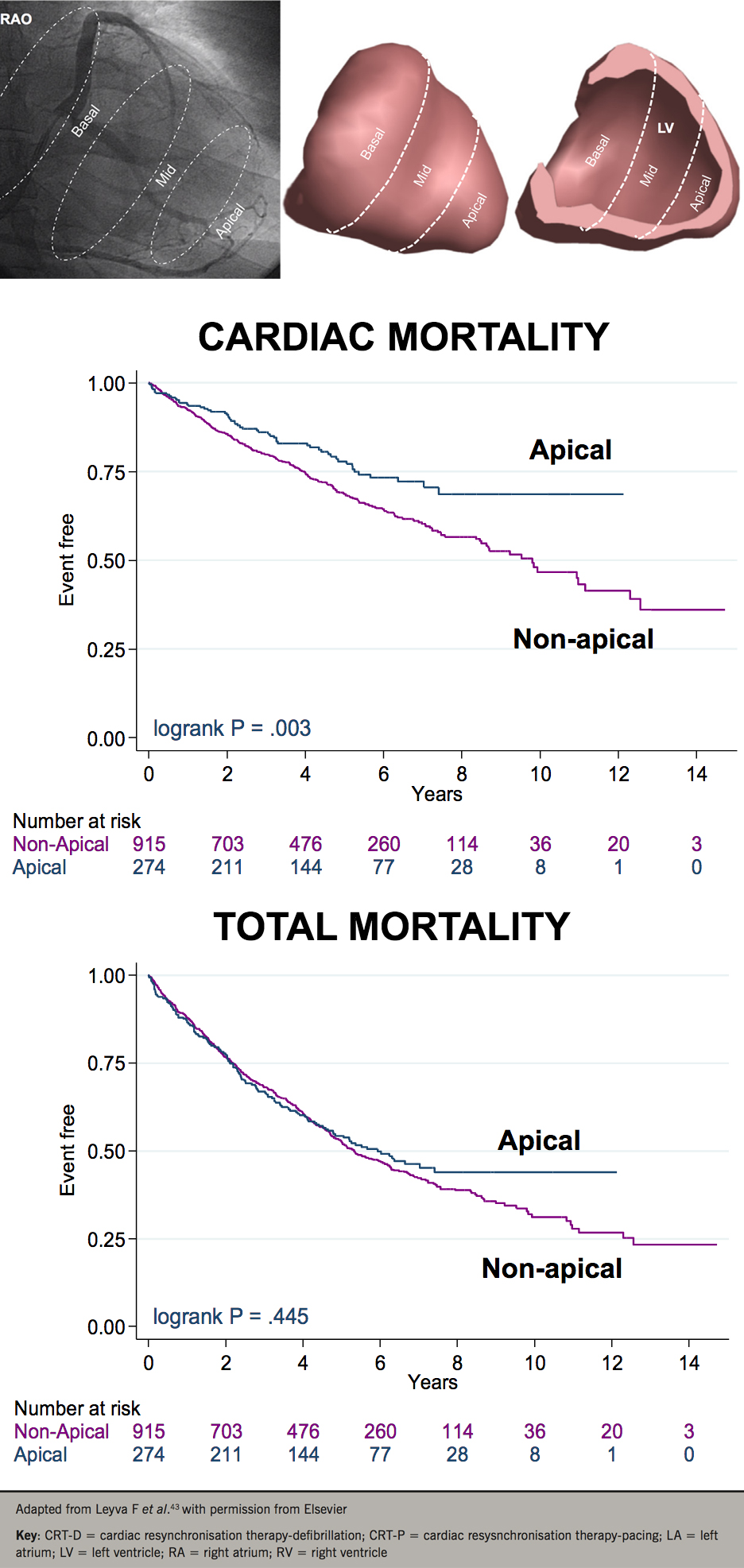
Apical or non-apical LV lead position?
It is now widely accepted that an apical LV position in CRT is undesirable. This notion emerged from subanalyses of MADIT-CRT38 and REVERSE,39 which are essentially observational studies, apical LV lead positions were less favourable than basal or mid-LV lead positions. Previous evidence, however, shows that an apical LV lead position is more favourable than non-apical positions.40-41 In humans, we observed a better haemodynamic response from LV apical pacing compared with basal LV pacing in patients with ICM and a LBBB.42 In a study of 1,189 patients undergoing CRT, we have shown that an apical LV lead position was associated with a lower cardiac mortality than a non-apical position, death from pump failure was lower with apical than with non-apical positions, as was the risk of SCD (66%) (figure 4).43 Despite recommendations from consensus statements, which are all based on observational data, it is still questionable whether an apical LV lead position in CRT is undesirable.
Device optimisation
Early CRT studies showed that atrioventricular delays govern LV function.44 Echocardiography gained credence in identifying which atrioventricular delay yields the optimum LV filling. Echocardiographic optimisation techniques, however, are laborious and impractical in routine clinical practice. Despite the fact that it was never tested or validated as an optimisation tool, echocardiographic optimisation was once regarded as the gold-standard. In RCTs, however, it appeared to be as effective as nominal device settings in ‘all-comers’.45 Nowadays, few centres undertake routine echocardiographic optimisation, choosing, perhaps, to do it only in ‘non-responders’.
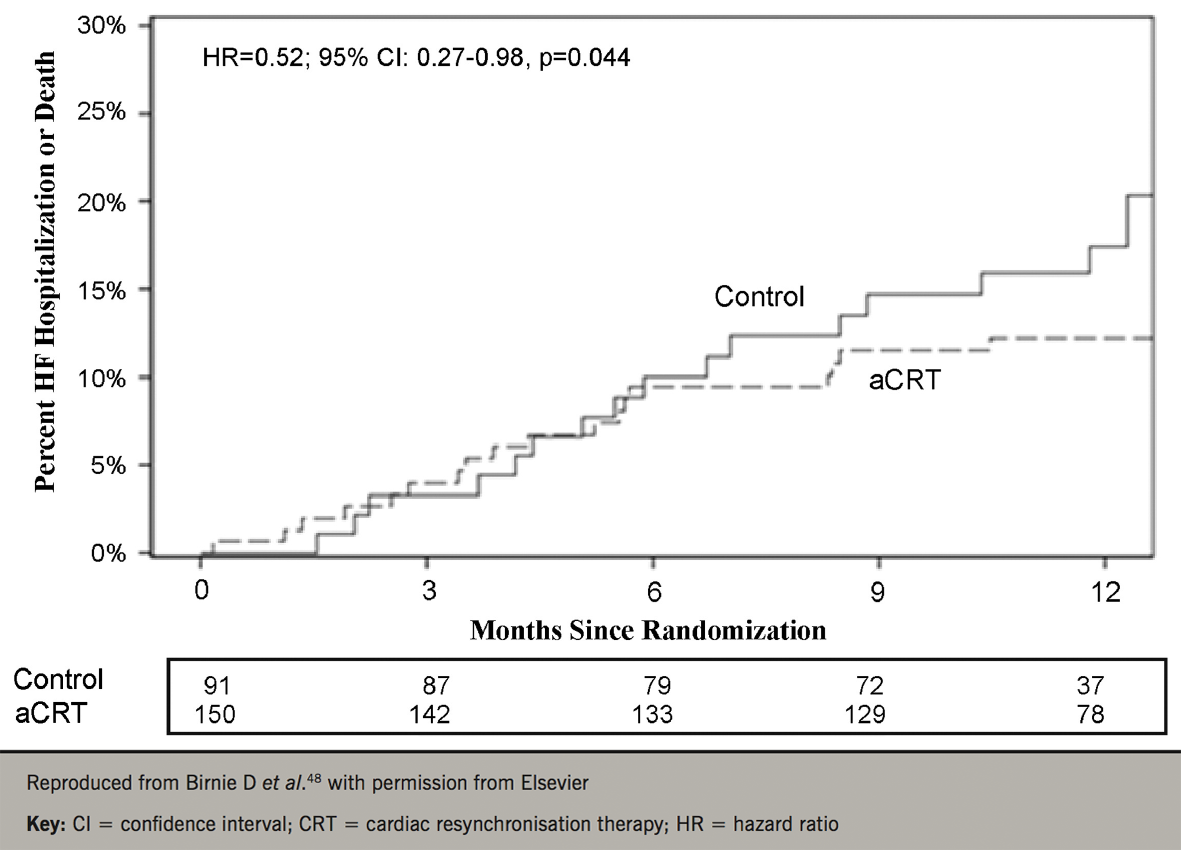
As the number of CRT implantations increases, so does interest in automatic, device-based optimisation. In the FREEDOM (A Frequent Optimization Study using the Quick Opt Method) study,46 QuickOpt was inferior to echocardiography. In the Smart-AV study (SD determined AV optimization; a comparison to other AV delay methods used in cardiac resynchronization therapy),45 Smart-AV algorithm did not lead to LV reverse remodelling, compared with nominal settings. A different strategy was provided by AdaptivCRT, which ‘titrates’ the amount of biventricular pacing delivered according to the intrinsic atrioventricular delay.47 This algorithm has been shown to lead to a better response to CRT, particularly in patients with a normal PR interval (figure 5).48
Targeting LV pacing sites: scar or latest activation?
Dyssychrony assessment using echocardiography was abandoned following the findings of the PROSPECT (Predictors of Response to Cardiac Resynchronization Therapy) study,49 in which no echocardiographic measure of dyssynchrony could reliably predict the response to CRT. The most negative aspect of dyssynchrony assessment in patient selection was provided by the Echo-CRT study, in which patients with a QRS <120 ms and with evidence of mechanical dyssynchrony derived no benefit from CRT.32 Accordingly, echocardiographic measures of dyssynchrony have been abandoned by all clinical guidelines.
There may still be a role for echocardiography in CRT in targeting LV pacing sites. In the TARGET (Targeted Left Ventricular Lead Placement to Guide Cardiac Resynchronization Therapy)50 and STARTER (Speckle Tracking Assisted Resynchronization Therapy for Electrode Region)51 studies, targeting late-activated segments appeared to improve outcomes. However, these studies also showed that myocardial scar, rather than latest mechanical activation, assessed using strain imaging, accounted for most of the effects of CRT. This is certainly in keeping with CMR studies, in which deploying the LV lead away from myocardial scar is associated with better outcomes.52,53
Multi-polar leads
Quadripolar LV leads were instantly welcomed by implanters, even before clinical evidence emerged in their favour. The possibility to use multiple pacing vectors to avoid phrenic nerve stimulation was a ‘game-changer’. Several studies have now shown that quadripolar leads are associated with higher implant success rates and lower rates of re-interventions for LV lead displacement or phrenic nerve stimulation.54,55 Surprisingly, quadripolar leads programmed to single-site LV pacing are also associated with better outcomes in observational studies.55,56 In the MORE-CRT (More Options Available With a Quadripolar LV Lead Provide In-Clinic Solutions to CRT Challenges) trial, in which 1,074 patients undergoing CRT-D were randomised to bipolar or quadripolar leads, freedom from the composite end point of intra-operative and post-operative LV lead-related events at six months was greater with QUAD than with bipolar leads (83.0% vs. 74.4%, p=0.0002). In a study of 847 CRT recipients, we showed that quadripolar leads were associated with a 68% lower total mortality.57 Re-interventions for LV lead displacement or phrenic nerve stimulation (PNS), which were lower with QUAD, predicted total mortality, cardiac mortality and HF hospitalisation.
Multi-site pacing and multi-point pacing
Studies on multiple LV leads might suggest that pacing from separate LV leads (multi-site pacing) may be preferable to pacing from a single lead. Leclercq et al. showed that multi-site pacing leads to a more pronounced LV remodelling response.58 However, implant success was relatively low and procedure times were high.
The possibility that multi-point pacing (MPP) might be superior to single-site LV pacing in CRT was explored by Lercher et al., who showed that, compared with atrial-only pacing, MPP was associated with a greater increase in systolic blood pressure than the best single-site configuration.59 Initial results from the MORE-CRT MPP (MOre REsponse on Cardiac Resynchronization Therapy With MultiPoint Pacing) study (NCT02006069) suggest that MPP may not be superior to single-site LV pacing in non-responders to CRT. Currently, MPP should be reserved as an option in non-responders, but certainly not before trying different LV vectors, and never in ‘all-comers’.
Endocardial pacing
Endocardial pacing in CRT is conceptually attractive, as the endocardial conduction is faster than epicardial conduction. Various modalities for LV endocardial pacing have emerged, using atrial transeptal and ventricular transeptal approaches. The worrying aspect of these approaches, however, is the risk of thromboembolic stroke and the difficulties that may ensue at extraction. The feasibility of endocardial stimulation for CRT with a leadless ultrasound-based technology has recently been shown,60 and outcome data are awaited. Importantly, however, this system relies on triggering from a pre-existing device, which means that patients will have been implanted with three devices and several leads. Combining such technology in a single device might be the way ahead.
The subcutaneous ICD and leadless CRT
The subcutaneous ICD (SICD) is a promising therapy for selected patients with indications for ICD therapy, particularly the young and those at risk of device infection. While RCTs comparing SICD and conventional transvenous ICDs are awaited, the SICD system is gaining popularity. Importantly, however, the current SICD cannot deliver CRT, nor bradycardia pacing. Efforts, however, are being made to combine the SICD with devices that deliver bradycardia pacing and CRT in a transvenous lead-free platform. Intuitively, a ‘modular’ platform that provides choice within a leadless CRT system and a SICD is attractive, but these are yet to emerge. For now, patients with a SICD who later require CRT-D are likely to undergo upgrade to a transvenous CRT-D system.
Personalised care
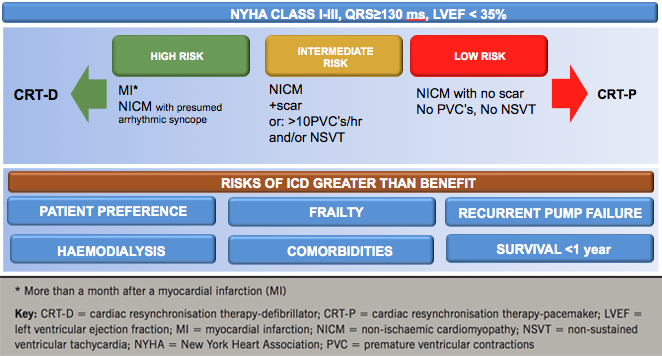
While clinical guidelines are the cornerstone of clinical decision-making, patient characteristics on which to base clinical decisions are limited. In CRT, they are NYHA class, LVEF, QRS duration and QRS morphology. The benefit of CRT-D and CRT-P, however, is also dependent on other factors, such as patient preference, frailty, renal function, comorbidities and life-expectancy in view of non-cardiac conditions, most of which have not been addressed by RCTs. Figure 6 shows a decision-making framework for use in deciding on device type, taking into account factors that have not been satisfactorily addressed in RCTs.
Conclusions
CRT is a very effective treatment for selected patients with severe and mild HF. Scrutiny of the findings of RCTs and numerous observational studies have provided useful insight as to which patients benefit the most. Technological advances, such as the quadripolar lead and LV endocardial systems have made CRT more effective and more widely available. The demonstration that device optimisation algorithms are as effective as echocardiography has also simplified follow-up. While indications for CRT are unlikely to change in the foreseeable future, the next 10 years may see the development of leadless CRT systems, perhaps in combination with a SICD.
Key messages
- Cardiac resynchronisation therapy (CRT) is very effective in a range of heart failure patients
- Quadripolar leads and left ventricular endocardial systems have made CRT more effective and widely available
- Leadless CRT systems, perhaps combined with subcutaneous implantable cardiac defibrillators (ICDs) may be developed in the future
Conflict of Interest
FL is a consultant and has received research support from Medtronic Inc, Abbott, Boston Scientific and Microport.
Articles in this supplement
Introduction
Current and future perspectives on cardiac pacing
A brief history of cardiac pacing in the UK
Drugs with devices in the management of heart failure
His-bundle pacing: UK experience and HOPE for the future!
Leadless pacing
Techniques in pacemaker and defibrillator lead extraction
Remote monitoring
Remote follow-up of ICS: a physiologist’s experience
References
1. Bakker P, Meijburg H, de Jonge N et al. Beneficial effects of biventricular pacing in congestive heart failure. Pacing Clin Electrophysiol 1994;17:820.
2. Cazeau S, Ritter P, Bachdach S et al. Four chamber pacing in dilated cardiomyopathy. Pacing Clin Electrophysiol 1994;17:1974–9. https://doi.org/10.1111/j.1540-8159.1994.tb03783.x
3. Leyva F, Nisam S, Auricchio A. 20 years of cardiac resynchronization therapy. J Am Coll Cardiol 2014;64:1047–58. https://doi.org/10.1016/j.jacc.2014.06.1178
4. Cleland JG, Daubert JC, Erdmann E et al. Longer-term effects of cardiac resynchronization therapy on mortality in heart failure [the CArdiac REsynchronization-Heart Failure (CARE-HF) trial extension phase]. Eur Heart J 2006;27:1928–32. https://doi.org/10.1093/eurheartj/ehl099
5. Woods B, Hawkins N, Mealing S et al. Individual patient data network meta-analysis of mortality effects of implantable cardiac devices. Heart 2015;101:1800–06. https://doi.org/10.1136/heartjnl-2015-307634
6. Colquitt J, Mendes D, Clegg A et al. Implantable cardioverter defibrillators for the treatment of arrhythmias and cardiac resynchronisation therapy for the treatment of heart failure: systematic review and economic evaluation. Health Technol Assess 2014;18. https://doi.org/10.3310/hta18560
7. Lindvall C, Chatterjee NA, Chang Y et al. National trends in the use of cardiac resynchronization therapy with or without implantable cardioverter-defibrillator. Circulation 2016;133:273–281. https://doi.org/10.1161/CIRCULATIONAHA.115.018830
8. Cunningham D, Charles R, Cunningham M, Whittaker T. Cardiac rhythm management. UK National Clinical Audit report 2013–2014. London: National Institute for Cardiovascular Outcomes Research, 2013. Available from: https://www.ucl.ac.uk/nicor/audits/cardiacrhythm/documents/annual-reports/CRM_National_Annual_Report_2013-14
9. Haugaa KH, Tilz R, Boveda S et al. Implantable cardioverter defibrillator use for primary prevention in ischaemic and non-ischaemic heart disease-indications in the post-DANISH trial era: results of the European Heart Rhythm Association survey. Europace 2017;19:660–4. https://doi.org/10.1093/europace/eux089
10. Kutyifa V, Geller L, Bogyi P et al. Effect of cardiac resynchronization therapy with implantable cardioverter defibrillator versus cardiac resynchronization therapy with pacemaker on mortality in heart failure patients: results of a high-volume, single-centre experience. Eur J Heart Fail 2014;16:1323–30. https://doi.org/10.1002/ejhf.185
11. Barra S, Boveda S, Providência R et al. Adding defibrillation therapy to cardiac resynchronization on the basis of the myocardial substrate. J Am Coll Cardiol 2017;69:1669–78. https://doi.org/10.1016/j.jacc.2017.01.042
12. Leyva F, Zegard A, Umar F et al. Long-term clinical outcomes of cardiac resynchronization therapy with or without defibrillation: impact of the aetiology of cardiomyopathy. Europace 2018. https://doi.org/10.1093/europace/eux357
13. Kober L, Thune JJ, Nielsen JC et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med 2016;375:1221–30. https://doi.org/10.1056/NEJMoa1608029
14. Al-Khatib SM, Fonarow GC, Joglar JA et al. Primary prevention implantable cardioverter defibrillators in patients with nonischemic cardiomyopathy: a meta-analysis. JAMA Cardiol 2017;2:685–8. https://doi.org/10.1001/jamacardio.2017.0630
15. Assomull RG, Prasad SK, Lyne J et al. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol 2006;48:1977–85. https://doi.org/10.1016/j.jacc.2006.07.049
16. Leyva F, Zegard A, Acquaye E et al. Outcomes of cardiac resynchronization therapy with or without defibrillation in patients with nonischemic cardiomyopathy. J Am Coll Cardiol 2017;70:1216–27. https://doi.org/10.1016/j.jacc.2017.07.712
17. Di Marco A, Anguera I, Schmitt M et al. Late Gadolinium enhancement and the risk for ventricular arrhythmias or sudden death in dilated cardiomyopathy: systematic review and meta-analysis. JACC Heart Fail 2016;5:28–38. https://doi.org/10.1016/j.jchf.2016.09.017
18. Wilkoff BL, Cook JR, Epstein AE et al. Dual-chamber pacing or ventricular back-up pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA 2002;288:3115–223. https://doi.org/10.1001/jama.288.24.3115
19. Sweeney MO, Hellkamp AS, Ellenbogen KA et al. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation 2003;107:2932–7. https://doi.org/10.1161/01.CIR.0000072769.17295.B1
20. Linde CM, Normand C, Bogale N et al. Upgrades from a previous device compared to de novo cardiac resynchronization therapy in the European Society of Cardiology CRT Survey II. Eur J Heart Fail 2018. https://doi.org/10.1002/ejhf.1235
21. Curtis AB, Worley SJ, Adamson PB et al. Biventricular pacing for atrioventricular block and systolic dysfunction. N Engl J Med 2013;368:1585–93. https://doi.org/10.1056/NEJMoa1210356
22. Leyva F, Zegard A, Patel K, Panting J, Marshall H, Qiu T. Clinical outcomes after upgrading from pacemakers to cardiac resynchronization therapy. Pacing Clin Electrophysiol 2018;41:290–8. https://doi.org/10.1111/pace.13287
23. Bristow MR, Saxon LA, Boehmer J et al. for the Comparison of Medical Therapy, Pacing and Defibrillation in Heart Failure (COMPANION) Investigators. Cardiac resynchronization therapy with or without an implantable defibrillator in advanced heart failure. N Engl J Med 2004;350:2140–50. https://doi.org/10.1056/NEJMoa032423
24. Cleland JGF, Daubert J-C, Erdmann E et al.; for the Cardiac Resynchronization-Heart Failure (CARE-HF) study investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005;352:1539–49. https://doi.org/10.1056/NEJMoa050496
25. Shen L, Jhund PS, Petrie MC et al. Declining risk of sudden death in heart failure. N Engl J Med 2017;377:41–51. https://doi.org/10.1056/NEJMoa1609758
26. Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: epidemiology, pathophysiology and rationale for therapy. Am J Cardiol 2003;91:2D–8D. https://doi.org/10.1016/S0002-9149(02)03373-8
27. Gasparini M, Leclercq C, Lunati M et al. Cardiac resynchronization therapy in atrial fibrillation patients: a multinational registry. The certify study. Eur Heart J 2013;34:48. https://doi.org/10.1093/eurheartj/eht307.48
28. Gasparini M, Kloppe A, Lunati M et al. Atrioventricular junction ablation in patients with atrial fibrillation treated with cardiac resynchronization therapy: positive impact on ventricular arrhythmias, implantable cardioverter-defibrillator therapies and hospitalizations. Eur J Heart Fail 2017. https://doi.org/10.1002/ejhf.1117
29. Yu CM, Chan YS, Zhang Q et al. Benefits of cardiac resynchronization therapy for heart failure patients with narrow QRS complexes and coexisting systolic asynchrony by echocardiography. J Am Coll Cardiol 2006;48:2251–7. https://doi.org/10.1016/j.jacc.2006.07.054
30. Achilli A, Sassara M, Ficili S et al. Long-term effectiveness of cardiac resynchronization therapy in patients with refractory heart failure and “narrow” QRS. J Am Coll Cardiol 2003;42:2117–24. https://doi.org/10.1016/j.jacc.2003.08.024
31. Foley PW, Patel K, Irwin N et al. Cardiac resynchronisation therapy in patients with heart failure and a normal QRS duration: the RESPOND study. Heart 2011;97:1041–7. https://doi.org/10.1136/hrt.2010.208355
32. Ruschitzka F, Abraham WT, Singh JP et al. Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N Engl J Med 2013;369:1395–405. https://doi.org/10.1056/NEJMoa1306687
33. Cleland JGF, Mareev Y, Linde C. Reflections on EchoCRT: sound guidance on QRS duration and morphology for CRT? Eur Heart J 2015;36:1948–51. https://doi.org/10.1093/eurheartj/ehv264
34. Gold MR, Thebault C, Linde C et al. Effect of QRS duration and morphology on cardiac resynchronization therapy outcomes in mild heart failure: results from the Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction (REVERSE) study. Circulation 2012;126:822–9. https://doi.org/10.1161/CIRCULATIONAHA.112.097709
35. Zareba W, Klein H, Cygankiewicz I et al. Effectiveness of Cardiac Resynchronization Therapy by QRS Morphology in the Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy (MADIT-CRT). Circulation 2011;123:1061–72. https://doi.org/10.1161/CIRCULATIONAHA.110.960898
36. Cleland JG, Abraham WT, Linde C et al. An individual patient meta-analysis of five randomized trials assessing the effects of cardiac resynchronization therapy on morbidity and mortality in patients with symptomatic heart failure. Eur Heart J 2013;34:3547–56. https://doi.org/10.1093/eurheartj/eht290
37. Kirkfeldt RE, Johansen JB, Nohr EA, Jørgensen OD, Nielsen JC. Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Denmark. Eur Heart J 2014;35:1186–94. https://doi.org/10.1093/eurheartj/eht511
38. Singh JP, Klein HU, Huang DT et al. Left ventricular lead position and clinical outcome in the multicenter automatic defibrillator implantation trial-cardiac resynchronization therapy (MADIT-CRT) trial. Circulation 2011;123:1159–66. https://doi.org/10.1161/CIRCULATIONAHA.110.000646
39. Thebault C, Donal E, Meunier C et al. Sites of left and right ventricular lead implantation and response to cardiac resynchronization therapy observations from the REVERSE trial. Eur Heart J 2012;33:2662–71. https://doi.org/10.1093/eurheartj/ehr505
40. van Deursen C, van Geldorp IE, Rademakers LM et al. Left ventricular endocardial pacing improves resynchronization therapy in canine left bundle-branch hearts. Circ Arrhythm Electrophysiol 2009;2:580–7. https://doi.org/10.1161/CIRCEP.108.846022
41. Durrer D, van Dam RT, Freud GE, Janse MJ, Meijler FL, Arzbaecher RC. Total excitation of the isolated human heart. Circulation 1970;41:899–912. https://doi.org/10.1161/01.CIR.41.6.899
42. Umar F, Taylor RJ, Stegemann B et al. Haemodynamic effects of cardiac resynchronization therapy using single-vein, three-pole, multipoint left ventricular pacing in patients with ischaemic cardiomyopathy and a left ventricular free wall scar: the MAESTRO study. Europace 2016;18:1227–34. https://doi.org/10.1093/europace/euv396
43. Leyva F, Zegard A, Taylor R et al. Long-term outcomes of cardiac resynchronization therapy using apical versus non-apical left ventricular pacing. J Am Heart Assoc 2018;7:3008508 (published online 14th August 2018) https://doi.org/10.1161/JAHA.117.008508
44. Auricchio A, Stellbrink C, Block M et al. Effect of pacing chamber and atrioventricular delay on acute systolic function of paced patients with congestive heart failure. The Pacing Therapies for Congestive Heart Failure Study Group. The Guidant Congestive Heart Failure Research Group. Circulation 1999;99:2993–3001. https://doi.org/10.1161/01.CIR.99.23.2993
45. Ellenbogen KA, Gold MR, Meyer TE et al. Primary results from the SmartDelay determined AV optimization: a comparison to other AV delay methods used in cardiac resynchronization therapy (SMART-AV) trial: a randomized trial comparing empirical, echocardiography-guided, and algorithmic atrioventricular delay programming in cardiac resynchronization therapy. Circulation 2010;122:2660–8. https://doi.org/10.1161/CIRCULATIONAHA.110.992552
46. Kamdar R, Frain E, Warburton F et al. A prospective comparison of echocardiography and device algorithms for atrioventricular and interventricular interval optimization in cardiac resynchronization therapy. Europace 2010;12:84–91. https://doi.org/10.1093/europace/eup337
47. Martin DO, Lemke B, Birnie D et al. Investigation of a novel algorithm for synchronized left-ventricular pacing and ambulatory optimization of cardiac resynchronization therapy: results of the adaptive CRT trial. Heart Rhythm 2012;9:1807–14. https://doi.org/10.1016/j.hrthm.2012.07.009
48. Birnie D, Lemke B, Aonuma K et al. Clinical outcomes with synchronized left ventricular pacing: analysis of the adaptive CRT trial. Heart Rhythm 2013;10:1368–74. https://doi.org/10.1016/j.hrthm.2013.07.007
49. Chung E, Leon A, Tavazzi L et al. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation 2008;117:2608–16. https://doi.org/10.1161/CIRCULATIONAHA.107.743120
50. Khan FZ, Virdee MS, Palmer CR et al. Targeted left ventricular lead placement to guide cardiac resynchronization therapy: the TARGET study: a randomized, controlled trial. J Am Coll Cardiol 2012;59:1509–18. https://doi.org/10.1016/j.jacc.2011.12.030
51. Saba S, Marek J, Schwartzman D et al. Echocardiography-guided left ventricular lead placement for cardiac resynchronization therapy: results of the Speckle Tracking Assisted Resynchronization Therapy for Electrode Region trial. Circ Heart Fail 2013;6:427–34. https://doi.org/10.1161/CIRCHEARTFAILURE.112.000078
52. Leyva F, Foley P, Chalil S et al. Cardiac resynchronisation therapy guided by late gadolinium-enhancement cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2011;13:29–35. https://doi.org/10.1186/1532-429X-13-29
53. Chalil S, Foley P, Muyhaldeen S et al. Late gadolinium enhancement-cardiovascular magnetic resonance as a predictor of response to cardiac resynchronization therapy in patients with ischaemic cardiomyopathy. Europace 2007;9:1031–7. https://doi.org/10.1093/europace/eum133
54. Forleo GB, Mantica M, Di Biase L et al. Clinical and procedural outcome of patients implanted with a quadripolar left ventricular lead: early results of a prospective multicenter study. Heart Rhythm 2012;9:1822–8. https://doi.org/10.1016/j.hrthm.2012.07.021
55. Behar JM, Bostock J, Zhu Li AP et al. Cardiac resynchronization therapy delivered via a multipolar left ventricular lead is associated with reduced mortality and elimination of phrenic nerve stimulation: long-term follow-up from a multicenter registry. J Cardiovasc Electrophysiol 2015;26:540–6. https://doi.org/10.1111/jce.12625
56. Turakhia MP, Cao M, Fischer A et al. Reduced mortality associated with quadripolar compared to bipolar left ventricular leads in cardiac resynchronization therapy. JACC: Clin Electrophysiol 2016;2:426–33. https://doi.org/10.1016/j.jacep.2016.02.007
57. Leyva F, Zegard A, Qiu T et al. Cardiac resynchronization therapy using quadripolar versus non-quadripolar left ventricular leads programmed to biventricular pacing with single-site left ventricular pacing: impact on survival and heart failure hospitalization. J Am Heart Assoc 2017;6 e007026. https://doi.org/10.1161/JAHA.117.007026
58. Leclercq C, Gadler F, Kranig W et al. A randomized comparison of triple-site versus dual-site ventricular stimulation in patients with congestive heart failure. J Am Coll Cardiol 2008;51:1455–62. https://doi.org/10.1016/j.jacc.2007.11.074
59. Lercher P, Lunati M, Rordorf R et al. Long-term reverse remodeling by cardiac resynchronization therapy with multipoint pacing: a feasibility study of noninvasive hemodynamics guided device programming. Heart Rhythm 2018;S1547-5271(18)30610-6. https://doi.org/10.1016/j.hrthm.2018.06.032
60. Auricchio A, Delnoy P-P, Butter C et al. Feasibility, safety and short-term outcome of leadless ultrasound-based endocardial left ventricular resynchronization in heart failure patients. Results of the Wireless Stimulation Endocardially for CRT (WiSE-CRT) study. Europace 2014;16:681–8. https://doi.org/10.1093/europace/eut435

