Recent studies have associated subclinical hypothyroidism with heart failure (HF) and increased mortality. To investigate the relationship between subclinical hypothyroidism and HF in Indian patients we prospectively recruited 350 HF patients between March 2013 and February 2017 at the department of cardiology Yashoda Hospital, Hyderabad, India. All patients underwent fasting serum glucose, lipid profile, N-terminal-pro-brain natriuretic peptide (NT-proBNP), and thyroid hormone levels. Risk factors and clinical evaluation were undertaken. We divided thyroid-stimulating hormone (TSH) levels into severity grade 1 (≤9.9 mIU/L) and grade 2 (≥10 mIU/L).
Out of 350 HF patients, 191 (54.5%) were men, mean age was 60.4 ± 10.2 years (range 36–85 years). The incidence of subclinical hypothyroidism was 18.5%, 69.4% had normal thyroid function, and 12% had overt hypothyroidism. Mean NT-proBNP levels were 3561 ± 5553 pg/mL and 10.5% suffered in-hospital mortality. Dyslipidaemia (p=0.004), elevated NT-proBNP levels (p<0.0001) and mortality (p<0.0001) were significantly associated with subclinical hypothyroidism compared with euthyroidism. After multi-variate analysis, hypertension (odds ratio [OR] 3.5; 95% confidence interval [CI] 2.32, 3.8), dyslipidaemia (OR 1.7; 95%CI 1.12, 2.8), subclinical hypothyroidism (OR 1.39; 95%CI 0.99, 1.82) and NT-proBNP >600 pg/mL (OR 1.98; 95%CI 1.23, 2.04) were significantly associated with HF. Grade 2 TSH (OR 4.16; 95%CI 2.04, 8.48), elevated NT-proBNP >1800 pg/mL (OR 2.18; 95%CI 1.53, 4.82), and severe left ventricular dysfunction (OR 2.51; 95%CI 1.24, 2.07) were significantly associated with poor outcome.
In conclusion, our study has established that subclinical hypothyroidism is associated with HF and grade 2 TSH has an independent association with in-hospital mortality in Indian patients.
Introduction
Heart failure (HF) is a complex disease, characterised by the reduced capacity of the heart to pump blood, supply or fill with blood, and is a cause of hospitalisation.1 HF is rapidly growing in developed and developing countries, with an estimated prevalence of more than 37 million individuals.2 HF is associated with a high rate of hospitalisation and it is a major cause of morbidity and mortality worldwide.1-3 Existing studies have shown that several comorbid factors and biomarkers are associated with HF and its prognosis.4 Recent studies have associated subclinical hypothyroidism with increased blood pressure, insulin resistance, endothelial dysfunction, oxidative stress, fatal stroke, elevated vascular stiffness, cardiovascular diseases and lipid levels.5 Subclinical hypothyroidism is defined by high levels of thyrotropin or thyroid-stimulating hormone (TSH) and normal levels of thyroxine.4 Studies have noted subclinical hypothyroidism is more common than other thyroid dysfunctions,1 and prevalence in the general population is between 4% and 20%.6 Subclinical hypothyroidism can affect the cardiovascular system via increased heart rate, and severe left ventricular dysfunction.2,6 TSH levels ≥10 mIU/L are associated with a high cardiovascular mortality rate4 and poor outcome in HF.3 The aim of this study was to investigate the relationship between subclinical hypothyroidism and HF in Indian patients. There are limited studies on this topic available from the Indian subcontinent.
Methods
We prospectively recruited 450 patients with HF admitted to the department of cardiology at Yashoda Hospital, Hyderabad. Among 425 patients, 25 patients refused tests, and within 24 hours, 50 patients left against medical advice (LAMA) or were discharged against medical advice (DAMA), the remaining 350 patients were included in the study. Yashoda Hospital is a tertiary care centre and teaching hospital in South India and the study took place between March 2013 and February 2017. All patients underwent fasting serum glucose, lipid profile, chest X-ray, blood chemistry, liver function test, kidney function test, N-terminal pro-brain natriuretic peptide (NT-proBNP), thyroid function tests, 12-lead electrocardiogram (ECG) and 2D echo.
Selection of cases
HF was defined based on clinical symptoms of breathlessness, ankle swelling and fatigue that may be accompanied by signs (e.g. elevated jugular venous pressure, pulmonary crackles and peripheral oedema) caused by a structural and/or functional cardiac abnormality, resulting in a reduced cardiac output and/or elevated intra-cardiac pressures at rest or during stress.3
Inclusion and exclusion criteria
All first presentation acute HF patients admitted to the department of cardiology, Yashoda Hospital, who were not taking thyroid medication were included in the study. The study excluded patients with present or past history of brain injury within three months, trauma, recurrent HF, severe kidney injury (creatinine plasma level above 4.9 mg/dL), estimated GFR index <35 ml/min/m2, liver disease, surgery, malignancy, pulmonary oedema, valvular heart disease, thyrotoxicosis, tachyarrhythmia, those who are currently pregnant, have implanted pacemakers, ischaemic stroke, intracranial haemorrhage, ischaemic events within the previous three months, acute infections, inflammatory conditions within the previous month, patients taking medications for hypo- or hyperthyroidism. Standardised questions were adopted from the behavioural risk factor surveillance system and risk factor assessment performed in all cases.7
Evaluation of left ventricular (LV) dysfunction
All patients underwent 2D echo, 12-lead ECG, and other routine investigations. Left ventricular (LV) dysfunction was divided into three categories (mild, moderate and severe). Mild LV dysfunction was ejection fraction between 41–45%, moderate LV dysfunction ejection fraction between 36–40% and severe LV dysfunction ejection fraction ≤35%.8
Estimation of thyroid hormones
Euthyroidism was defined as a serum TSH level of 0.35–5.50 µIU/ml with normal fT4 (thyroxine) concentration (0.89–1.76 ng/dL). Overt hypothyroidism was defined as a serum fT4 concentration <0.89 ng/dL with serum TSH level >5.50 µIU/ml. Subclinical hypothyroidism was defined as a serum TSH level of >5.50 µIU/ml with normal fT4 concentrations.3 Subclinical hypothyroidism was divided, based on severity, into grade 1 TSH (TSH levels ≤9.9 mIU/L) and grade 2 TSH (TSH ≥10 mIU/L).
Analysis of NT-proBNP
In our laboratory we used ADVIA Centaur CP BNP assay (Siemens AG, Munich, Germany), and NT-proBNP up to 5,000 pg/ml. This NT-proBNP instrument’s sensitivity is 81.4–92.0% and specificity 62.1–96.8%. In our lab manual and current literature NT-ProBNP ≥600 pg/ml were considered abnormal.9
Assessment of risk factors
Hypertension was diagnosed as a systolic blood pressure more than 140 mmHg and/or a diastolic blood pressure more than 90 mmHg based on the average of two blood pressure measurements or a patient’s self-reported history of hypertension. Alcoholics were defined by an alcohol intake more than 50 g/day (equivalent to 500 ml [two glasses] of wine, 1,000 ml of beer, or five drinks [units] of spirits). Diabetes mellitus was diagnosed if the fasting plasma glucose was more than ≥126 mg/dL or patients were on antidiabetic medications. Smokers were defined as patients reporting daily smoking. Dyslipidaemia was defined as one or more of the following: total cholesterol more than 200 mg/dL, low-density lipoprotein-cholesterol (LDL-C) more than 130 mg/dL, high-density lipoprotein-cholesterol (HDL-C) below 40 mg/dL, very-low-density lipoprotein-cholesterol (VLDL-C) more than 30 mg/dL, triglycerides more than 150 mg/dL.7
Statistical analysis
Statistical analysis was performed using SPSS 16.0 windows software (Statistical Package for the Social Sciences, SPSS Inc.). Continuous variables were performed in titre of mean ± standard deviation (SD). Categorical variables were expressed as proportions and chi-square test was used to study the association between proportions. Univariate and multi-variate analysis was performed for potential confounders. All tests were two-sided and p value <0.05 was considered statistically significant.
Results
In this study, 191 (54.5%) were male, age range 36–85 years and the mean age was 60.4 ± 10.2 years. Subclinical hypothyroidism was found in 65 (18.5%) patients, overt hypothyroidism in 42 (12%) and euthyroidism in 243 (69.4%). Mean NT-proBNP was 3561 ± 5553 pg/ml in HF. The overall in-hospital mortality was 37 (10.5%).
Table 1 shows the comparison of values in those with hypothyroidism versus euthyroid. Significant associations were found for dyslipidaemia (p=0.004), acute HF (p=0.0005), mean NT-proBNP levels (p=0.04) and mortality (p<0.0001) in subclinical hypothyroidism compared with euthyroidism in HF. Mean total cholesterol, LDL-C, HDL-C, VLDL-C and triglycerides were significantly associated with subclinical hypothyroidism (p<0.0001) compared with euthyroidism.
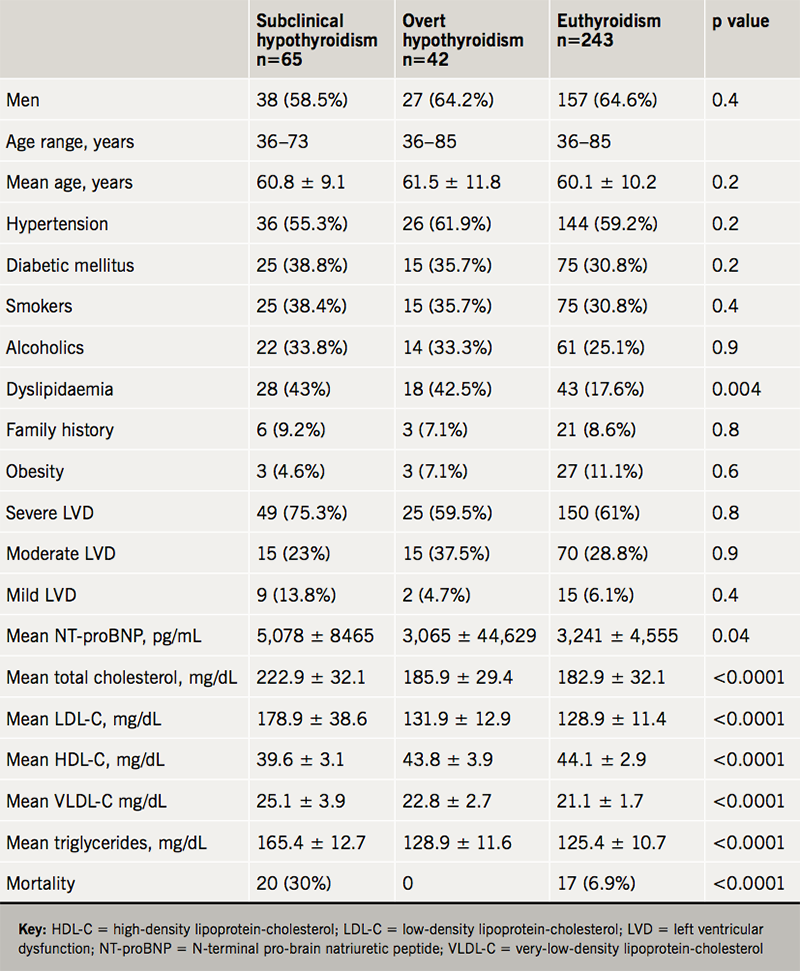
In the multi-variate analysis, hypertension (odds ratio [OR] 3.50; 95% confidence interval [CI] 2.32, 3.80), diabetes (OR 2.83; 95%CI 1.65, 3.26), smoking (OR 1.72; 95%CI 1.01, 2.12), NT-proBNP >600 pg/ml (OR 1.98; 95%CI 1.23, 2.04) and subclinical hypothyroidism (OR 1.39; 95%CI 0.99, 1.82) were significantly associated with HF. The independent predictors of mortality in hospital were grade 2 TSH (OR 4.16; 95%CI 2.04, 8.48), NT-proBNP ≥1,801 pg/ml (OR 2.18; 95%CI 1.53, 4.82) and severe LV dysfunction (OR 2.51; 95%CI 1.24, 2.07) (table 2).

Discussion
In our study, we found 18.5% of HF patients had subclinical hyperthyroidism. Other studies have shown similar findings: Hayashi et al. 21%,4 Berezin et al. 13.6%,10 Gencer et al. 8.1%,11 and Walsh et al. 5.6%.12
Our study establishes that subclinical hypothyroidism is associated with HF (OR 1.39; 95%CI 0.99, 1.82). Our findings support those of Berezin et al. (OR 1.22; 95%CI 1.17, 1.32)10 and Hayashi et al. (OR 2.31; 95%CI 1.44, 3.67).4
Thyroid hormones play a vital role in the homeostasis of the cardiovascular system, and have effects on cardiac contractility, cardiac output, vascular resistance blood pressure and decreased LV systolic function on exercise. Higher TSH levels among participants with subclinical hypothyroidism have been correlated with a decreased LV stroke volume; reduction in the cardiac index, an increase in systemic vascular resistance and HF.11 However, some studies found no significant association.12
In our study, we found elevated total cholesterol, LDL-C, VLDL-C, triglycerides and low levels of HDL-C were significantly associated with subclinical hypothyroidism in HF (p<0.0001), findings supported by others.13,14
We also found that age (>65 years) and gender were not significantly associatied with HF, findings supported by previous studies.4 However, a few studies have noted that age >65 years,7,16 male12 and female gender13 were significantly associated with HF.
We established hypertension was independently associated with HF (OR 3.5; 95%CI 2.3, 3.8). Our findings are in concordance with Rani et al. who showed hypertension increased the risk of HF by 3.8 times.16 The possible mechanism of HF involves both a combination of systolic and diastolic dysfunction and changes in LV systolic and diastolic function, and progress of ventricular hypertrophy and development of HF.16
We also established that diabetes is independently associated with HF (OR 2.83; 95%CI 1.65, 3.26), findings advocated by Bauters et al. (relative risk [RR] 1.37; 95%CI 1.21, 1.55).17 However, a recent study showed diabetic mellitus had no significant association with HF.10 The mechanism of diabetic mellitus in HF is thought to involve LV concentric remodelling associated with impaired myocardial energetics and reduced systolic strain. The hypertrophy of the diabetic heart is the consequence of myocardial triglyceride deposition and increased extracellular volume (collagen deposition and fibrosis). In addition, hyperinsulinaemia due to insulin resistance also directly promotes myocardial hypertrophy, energetic substrate availability, oxygen supply myocardial tissue perfusion, microcirculatory damage and is a cause for diabetic cardiomyopathy.18
Our study showed that NT-proBNP (>600 pg/ml) was significantly associated with HF (OR 1.98; 95%CI 1.23, 2.04), also shown in another study (>400 pg/ml: OR 1.19; 95% CI 1.12, 1.25).10
In our study, we found no significant association between dyslipidaemia and HF (OR 0.41; 95%CI 0.06, 0.80). Other studies have shown similar findings.15 However, Berezin et al. showed dyslipidaemia to have an independent association with HF (OR 1.06; 95%CI 1.04, 1.11).10
We found cigarette smoking to be significantly associated with HF (OR 1.72; 95%CI 1.01, 2.12) supporting Gopal et al. (hazard ratio [HR] 1.73; 95%CI 1.15, 2.59).19 The mechanism by which smoking promotes atherosclerosis appears to be mediated by inflammation and risk of HF. Cigarette smoking directly increases free radical production, cardiac mitochondrial damage, nicotine-induced free fatty acid release and increases the development of HF.19
Our study, we noted overall in-hospital mortality was 10.5%, and 30% in subclinical hypothyroidism with HF. We established grade 2 TSH to be independently associated with mortality (OR 4.16; 95%CI 2.04, 8.48). Our findings are supported by others,4,12 and we found no significant association of grade 1 TSH with HF, in agreement with others.3
Recent studies have found elevated NT-proBNP (levels ≥1,800 pg/ml) are significantly associated with hospital mortality and poor outcome.20 Our study also established NT-proBNP was independently associated with in-hospital mortality (OR 2.18; 95%CI 1.53, 4.82) these findings are supported by Berin et al. (HR 3.7; 95%Cl 1.20, 11.8).20
In our study, we found severe LV dysfunction to be an independent predictor of mortality in HF patients (OR 2.51; 95%CI 1.24, 2.07). Our findings support Bhakta et al. (OR 3.9; 95%CI 2.3, 6.4).21
Clinical implications
The American Heart Association recommends thyroid function testing to investigate exacerbation of HF and that replacement doses of both T3 and T4 reduce the risk of HF. Recent clinical trials have demonstrated grade 2 TSH treatment with L-T4 for 48 weeks provides a significant reduction of LDL-C levels.22
Study limitations
The present study has limitations. First, thyroid function was measured at baseline, and the possible progression from subclinical to overt dysfunction was unknown, as well as whether a raise of TSH level on admission for HF had been pre-existing before developing HF. This limitation is common to all published observational studies.11 Second, we were unable to perform analysis of HF subtypes, or aetiology of HF with subclinical hypothyroidism.
Conclusion
Our study shows that subclinical hypothyroidism is significantly associated with HF. Grade 2 TSH, severe LV dysfunction and elevated NT-proBNP levels (>1,800 pg/ml) were independently associated with mortality. Our study noted that elevated cholesterol levels were significantly associated with subclinical hypothyroidism in HF. Randomised-controlled studies are required to evaluate the efficacy of L-T4 administration in preventing the risk of HF. Larger-scale multi-centre studies are required to establish the potential role of these findings in HF management.
Key messages
- Our study found subclinical hypothyroidism in 18.5% of Indian heart failure patients
- Our study noted 30% of mortality in subclinical hypothyroidism with heart failure
- Our study found grade 2 thyroid-stimulating hormone (TSH ≥10 mIU/L) to be an independent predictor of poor outcome in heart failure
- Our study showed elevated N-terminal pro-brain natriuretic peptide levels (NT-proBNP ≥600 pg/mL) to be significantly associated with heart failure
Conflicts of interest
None declared.
Acknowledgements and funding
The authors are thankful to Dr G S Rao, Managing Director, and Dr A Lingaiah, Director of Medical Services, Yashoda group of hospitals, for their generous support to carry out this study in Yashoda Hospital, Hyderabad.
Study approval
This study was approved by the institutional ethics committee of Yashoda Hospital.
Consent
Consent was obtained from patients.
References
1. Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev 2017;3:7–11. https://doi.org/10.15420/cfr.2016:25:2
2. Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nature Rev Cardiol 2016;13:368–78. https://doi.org/10.1038/nrcardio.2016.25
3. Hassan A, Altamirano-Ufion A, Zulfiqar B et al. Sub-clinical hypothyroidism and its association with increased cardiovascular mortality: call for action. Cardiol Res 2017;8:31–5. https://doi.org/10.14740/cr524w
4. Hayashi T, Hasegawa T, Kanzaki H et al. Subclinical hypothyroidism is an independent predictor of adverse cardiovascular outcomes in patients with acute decompensated heart failure. ESC Heart Fail 2016;3:168–76. https://doi.org/10.1002/ehf2.12084
5. Sun J, YaoL, Fang Y et al. Relationship between subclinical thyroid dysfunction and the risk of cardiovascular outcomes: a systematic review and meta-analysis of prospective cohort studies. Int J Endocrinol 2017;2017:8130796. https://doi.org/10.1155/2017/8130796
6. Moon S, Kim MJ, Yu JM et al. Subclinical hypothyroidism and the risk of cardiovascular disease and all-cause mortality: a meta-analysis of prospective cohort studies. Thyroid 2018;28:1101–10. https://doi.org/10.1089/thy.2017.0414
7. Bandaru VCS, Kaul S, Laxmi V et al. Antibodies to Chlamydia pneumoniae are associated with increased intima media thickness in asymptomatic individual Indian patients. J Stroke Cerebrovasc Dis 2009;18:190–4. https://doi.org/10.1016/j.jstrokecerebrovasdis.2008.09.020
8. Raymond I, Pedersen F, Steensgaard-Hansen F et al. Prevalence of impaired left ventricular systolic function and heart failure in a middle aged and elderly urban population segment of Copenhagen. Heart 2003;89:1422–9. https://doi.org/10.1136/heart.89.12.1422
9. Troughton RW, Richards AM. B-type natriuretic peptides and echocardiographic measures of cardiac structure and function. JACC Cardiovasc Imaging 2009;2:216–25. https://doi.org/10.1016/j.jcmg.2008.12.006
10. Berezin AE, Kremzer AA, Martovitskaya YV et al. The association of subclinical hypothyroidism and pattern of circulating endothelial-derived microparticles among chronic heart failure patients. Res Cardiovasc Med 2015;4:e29094. https://doi.org/10.5812/cardiovascmed.29094
11. Gencer B, Collet TH, Virgini V et al. Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from six prospective cohorts. Circulation 2012;126:1040–9. https://doi.org/10.1161/CIRCULATIONAHA.112.096024
12. Walsh JP, Bremner AP, Bulsara MK et al. Subclinical thyroid dysfunction as a risk factor for cardiovascular disease. Arch Intern Med 2005;165:2467–72. https://doi.org/10.1001/archinte.165.21.2467
13. Maleki N, Kazerouni F, Hedayati M et al. Subclinical hypothyroidism and the alterations of lipid profile as a cardiovascular risk factor. J Paramed Sci 2015;6:20–5. Available at: http://journals.sbmu.ac.ir/jps/article/viewFile/10623/8123
14. Hernández-Mijares A, Jover A, Bellod L et al. Relation between lipoprotein subfractions and TSH levels in the cardiovascular risk among women with subclinical hypothyroidism. Clin Endocrinol 2013;78:777–82. https://doi.org/10.1111/cen.12064
15. Rodondi N, Bauer DC, Cappola AR et al. Subclinical thyroid dysfunction, cardiac function, and the risk of heart failure. The Cardiovascular Health study. J Am Coll Cardiol 2008;52:1152–9. https://doi.org/10.1016/j.jacc.2008.07.009
16. Rani NV, Kannan G, Vasantha J et al. Risk assessment for congestive heart failure in a South Indian population: a clinical pharmacist’s perspective. Indian Journal of Clinical Practice 2012;22:431–4. Available at: http://medind.nic.in/iaa/t12/i2/iaat12i2p431.pdf
17. Bauters C, Lamblin N, McFadden EP et al. Influence of diabetes mellitus on heart failure risk and outcome. Cardiovasc Diabetol 2003;2:1. https://doi.org/10.1186/1475-2840-2-1
18. Lehrke M, Marx N. Diabetes mellitus and heart failure. Am J Med 2017;130:S40–S50. https://doi.org/10.1016/j.amjmed.2017.04.010
19. Gopal DM, Kalogeropoulos AP, Georgiopoulou VV et al. Cigarette smoking exposure and heart failure risk in older adults: the Health, Aging, and Body Composition study. Am Heart J 2012;164:236–42. https://doi.org/10.1016/j.ahj.2012.05.013
20. Berin R, Zafrir B, Salman N et al. Single measurement of serum N-terminal pro-brain natriuretic peptide: the best predictor of long-term mortality in patients with chronic systolic heart failure. Eur J Intern Med 2014;25:458–62. https://doi.org/10.1016/j.ejim.2014.04.001
21. Bhakta D, Groh MR, Shen C et al. Increased mortality with left ventricular systolic dysfunction and heart failure in adults with myotonic dystrophy type 1. Am Heart J 2010;160:1137–41. https://doi.org/10.1016/j.ahj.2010.07.032
22. Feldt-Rasmussen U. Subclinical hypothyroidism and cardiovascular risk – an overview of current understanding. Eur Endocrinol 2011;7:53–7. https://doi.org/10.17925/EE.2011.07.01.53
