Implantable cardiac monitors (ICMs), also known as implantable loop recorders (ILRs), are used for long-term heart rhythm monitoring of unexplained syncope or in the detection of arrhythmias. These devices are implanted by cardiologists within a cardiac catheter suite environment. The newer generation devices are miniaturised and inserted using a specific tool kit via a minimally invasive procedure. This paper describes the changes we have made to allow these devices to be implanted in a non-theatre environment by a cardiac physiologist and the benefits and cost reduction of this service redesign.
A cardiac physiologist (LC, Band 6) undertook specific training beginning in September 2015. A standard operating procedure (SOP) was developed and patient information videos were commissioned. The new service was introduced in September 2016 in the screening room of our critical care unit (CCU). Data were collected prospectively on the clinical outcome, patient satisfaction and costs.
Over a 13-month period LC independently performed 116 procedures (113 Medtronic Reveal LINQ™ ICMs and 3 St. Judes SJM CONFIRM™) with only one minor complication. Patients were highly satisfied with the redesigned service, which showed a reduction in cost of £241.27 per case.
ICMs/ILRs can be implanted safely and cost-effectively outside a cardiac catheter suite environment by a cardiac physiologist. This requires some specific training, a clinical SOP and is supported by use of dedicated patient information videos.
Introduction
Implantable cardiac monitors (ICMs) are becoming more commonly recommended during the investigation of a patient with transient loss of consciousness.1,2 The original first-generation devices were implanted by a surgical procedure, usually by a cardiologist in a cardiac catheterisation theatre on a day-case basis, lasting up to 30–45 minutes per procedure. This process is inherently resource heavy for theatre time, staff (implanting physician, catheter lab nurses, cardiac radiographer and cardiac physiologist), in addition to pre-admission assessment, surgical equipment and patient recovery in a day-case facility. The development of the next generation miniaturised Reveal LINQTM ICM3 device (Medtronic plc, Dublin, Ireland) provides the possibility of performing these procedures with a more simplified insertion kit over 20 minutes, as well as requiring less staff and with lower cost.1,4 We describe how we trained one of our cardiac physiologists (LC) to implant ICM devices autonomously in the screening room of our coronary care unit, and discuss the clinical benefits and cost savings of this service redesign.
Methodology
Operator training
Several challenges needed to be overcome to permit a cardiac physiologist to perform LINQ™ implants. In September 2015, LC (cardiac physiologist, Band 6 with an aim to reaching Band 7 once implanting independently) began in-house training to equip her with the necessary skills to autonomously implant LINQ™ devices. This entailed her undertaking the following training:
- Mental Capacity Act
- Patient consent
- European Resuscitation Council advanced life support (ALS)
- Intravenous cannulation
- Intravenous drug administration
- Surgical skills – suturing course (Royal College of Surgeons).
Furthermore, a stepwise process was then followed leading to LC implanting LINQ™ implants independently:
- Review LINQ™ implantation video (Medtronic).5
- Undertook Mental Capacity Act/informed consent course (online, Trust approved).
- Undertook training on use and administration of local anaesthetic mediated by a Trust pharmacist and an experienced implanter physician supervisor (PJK). It was necessary to amend the Trust’s medicines management policy to permit a clinical physiologist to administer medication, namely local anaesthetic. Clinical physiologists are classified as a voluntary registered healthcare professional and are not legally permitted to administer medications under patient group directions (PGDs) but are permitted to administer against a named patient prescription order. Thus, to allow a cardiac physiologist to administer local anaesthetic there are two options; adapt the request form into a prescription or use an independent prescriber to prescribe the medication prior to the procedure.
- Shadowed an experienced implanter (PJK) during three implant procedures – this allowed LC to become familiar with practical aspects of the procedure including how to explain the procedure to the patient, how to obtain fully informed consent, and how to undertake the necessary pre-procedural checks, as well as the actual implantation procedure.
- Performed 50 procedures with support from a cardiologist. A logbook was maintained for every procedure and formal feedback provided after each case. Surgical scrub training was led by a scrub nurse and recorded in LC’s logbook.
- Sign off authorising LC as independent implanter (September 2016). Her job description was amended to include LINQ™ implantation to ensure compliance with NHSLA (National Health Service Litigation Authority) requirements.
- Following this, LC has now also commenced implanting St. Jude Confirm devices (so far three cases).
Service redesign
We decided to review the current implantation process originally introduced to implant first generation devices and redesign the service for the new LINQ™ device. Key service developments were:
- A standard operating procedure (SOP) (table 1).
- Educational videos – we made two educational videos in association with Health and Care Videos (http://videos.torbayandsouthdevon.nhs.uk/cardiology/17751).6 The first describes what will happen on the day of the procedure from a patient’s point of view including what they need to do in preparation, as well as information about the implantation itself. The second describes the use of the “MyCarelink™” remote communicator and how this should be set up at home and used for on-demand recordings. Both were made available via our trust website and on a tablet device for those patients who did not have internet access.
- A dedicated LINQ™ implant list (monthly). From November 2015 all patients referred for LINQ insertion were booked into a monthly implantation (first list 10 December 2015). Patients were allocated 20-minute slots with a maximum of eight patients per list. Patients were asked to attend the waiting area fully dressed but wearing buttoned shirts/blouses, which could easily be undone. Warfarin and direct oral anticoagulants (DOACs) were not suspended, but those patients on warfarin required near-patient testing (NPT) international normalised ratio (INR) tests prior to implant (see SOP, table 1). Anxious patients were able to have their spouse/relative/friend accompany them during the procedure. After device implantation we initially undertook individual training on the use of the home monitor, but this quickly developed into group sessions of three to four patients together with their attending spouses/relatives/friends.

Results
Clinical
LC has now performed 116 ICM implant procedures (113 LINQ™ and three St. Jude Confirm™ devices). Two patients developed minor bleeding, which required the use of a hand-held diathermy (both were on anticoagulants). Two patients required skin sutures to adequately close the wound. No patient developed early or late infection or erosion of the device. Eight of these patients had permanent pacemakers (PPMs) already in situ with no complications from ICM insertion. The only significant complication was a small apical pneumothorax related to deep anaesthetic infiltration in a patient with very low body mass index (BMI, 18 kg/m2). This patient was monitored overnight and discharged the next day. Patient feedback was obtained from the first 25 patients and has been highly supportive of the new service and was without any significant criticism.
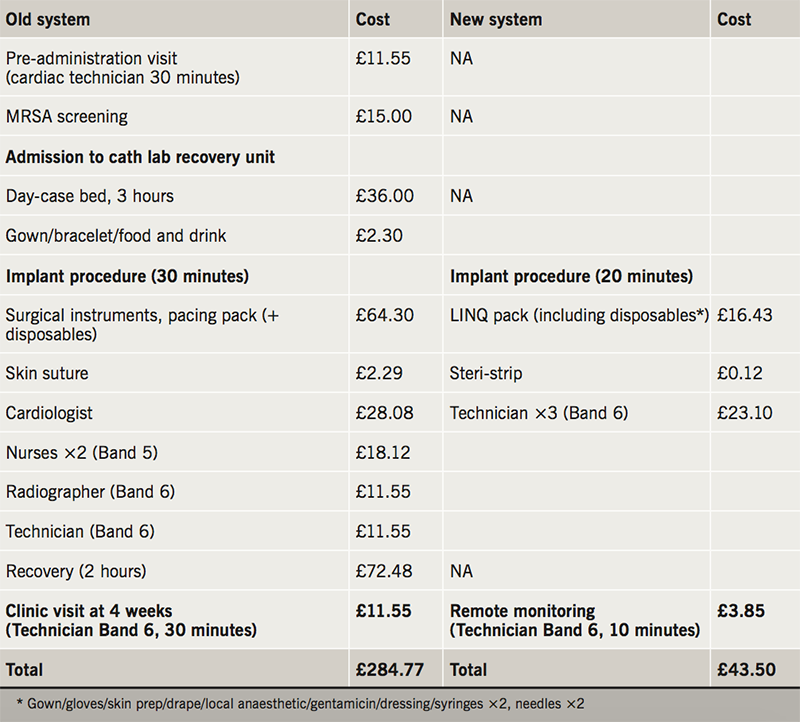
Costings
Table 2 details the healthcare costs of the old and new ICM implantation systems at our centre. This demonstrates a net saving amount of £241.27 per implant procedure using the new system.
Discussion
The use of ICMs is set to markedly increase, with their use earlier in the investigative algorithm for syncope and with our ageing population. There is, thus, a pressing need to perform these procedures efficiently and cost-effectively. Device miniaturisation provides an opportunity to redesign the traditional service of a cardiologist implanting the device in a cardiac catheterisation theatre.
In this report, we have shown ICM devices can be safely and routinely implanted in a non-theatre environment.3 This is currently under study in the US in the Reveal In-Office 2 (RIO 2) study (NCT 02395536).8 A recent health-economic analysis in three European countries hypothesised savings of 600–800 Euros per case by moving the procedure out of a cardiac catheter laboratory.7 Our experience, in the real world, confirms a cost saving of £241.27 per case.
In addition to the financial savings of this service redesign, there are clearly additional benefits in moving these procedures out of the cardiac catheterisation theatre, thereby freeing up staff and facilities for other activity. In the next 12 months we anticipate performing 100 ICM device implants in our screening room, thereby enabling 17 additional 30-minute sessions of activity in the vacated cardiac catheterisation theatre. Removing this unnecessary activity from a cardiac catheterisation theatre is clearly particularly important for centres that operate using a single theatre and provide emergency procedures, such as coronary angioplasty.
However, the more innovative aspect of our new service is that we have trained a non-physician to perform these procedures. Procedural activity has traditionally been the province of a medically qualified individual, and achieving this for a non-physician required considerable time and effort on behalf of the trainee (LC) in undertaking the relevant training courses. The service redesign was greatly facilitated by the development of a clear and concise SOP document. In addition, we found that the development and use of the patient information videos markedly improved patient understanding and improved the consent process, allowing us to cancel any pre-admission review. We aim to further develop non-physician implantation by adding use of skin sutures and hand-held diathermy into the training regimen and have already started to train a second operator (MG), who has so far implanted four LINQ™ devices.
We believe redesigning ICM implantations to be undertaken by a non-physician in a non-theatre environment should become routine practice. This change in practice is becoming increasingly prominent,9,10 however, we have shown that a formal service redesign is possible and has led to better patient experience and satisfaction, generated more cardiac catheterisation theatre availability, as well as physician time availability, at a reduced overall cost.
Conflicts of interest
None declared.
Funding
Medtronic helped fund the video production.
Study approval
The article is focused on a service redesign for already provided procedures for patients, focused on a change in practice rather than new techniques or major research requiring human subjects, therefore, no ethics committee approval was requested as it was not required.
Key messages
- Loop recorders are recommended for the investigation of a patient with transient loss of consciousness. Conventionally, cardiologists insert loop recorders within a cardiac catheter suite environment. This is a time-consuming, resource-intensive and relatively expensive affair
- We suggest a cost-effective way of utilising the resources more efficiently and inserting loop recorders safely by a trained cardiac physiologist. This involved specific training, a clinical standard operating procedure and use of dedicated patient information videos
- If this became routine practice it could free up physicians’ time, as well as cut down on the running costs of cardiac catheter labs and will allow loop recorders to be inserted at a reduced overall cost to the service, as well as making it a more easily tolerated process by the patients and broadening the skills of cardiac physiologists
References
1. National Institute for Health and Care Excellence. Transient loss of consciousness (‘blackouts’) in over 16s. CG109. London: NICE, August 2010. Available from: https://www.nice.org.uk/guidance/cg109
2. Moya A, Sutton R, Ammirati F et al. Guidelines for the diagnosis and management of syncope (2009): the Task Force for the Diagnosis and Management of Syncope of the European Society of Cardiology (ESC). Eur Heart J 2009;30:2631–71. https://doi.org/10.1093/eurheartj/ehp298
3. Pürerfellner H, Sanders P, Pokushalov E et al. Miniaturized Reveal LINQ insertable cardiac monitoring system: first-in-human experience. Heart Rhythm 2015;12:1113–19. https://doi.org/10.1016/j.hrthm.2015.02.030
4. Steffel J, Wright DJ, Schäfer H, Rashid-Fadel T, Lewalter T. Insertion of miniaturized cardiac monitors outside the catheter operating room: experience and practical advice. Europace 2017;19:1624–9. https://doi.org/10.1093/europace/euw304
5. Medtronic. Reveal LINQ ICM system: our monitors. Available at: http://www.medtronic.com/patients/fainting/device/our-insertable-cardiac-monitors/reveal-LINQ-icm/
6. Torbay and South Devon NHS Foundation Trust. Linq insertable cardiac monitors. Available at: http://videos.torbayandsouthdevon.nhs.uk/cardiology/17751
7. Kanters TA, Wolff C, Boyson D et al. Cost comparison of two implantable cardiac monitors in two different settings: Reveal XT in a catheterization laboratory vs. Reveal LINQ in a procedure room. Europace 2016;18:919–24. https://doi.org/ 10.1093/europace/euv217
8. Health Research Authority. Reveal LINQ™ In-Office 2 (RIO 2) International study. Available at: https://www.hra.nhs.uk/planning-and-improving-research/application-summaries/research-summaries/reveal-linqtm-in-office-2-rio-2-international-study/
9. Roebuck A, Mercer C, Denman J, Houghton AR, Andrews R. Experiences from a non-medical, non-catheter laboratory implantable loop recorder (ILR) service. Br J Cardiol 2015;22:36. https://doi.org/10.5837/bjc.2015.004
10. Davis S, Westby M, Pitcher D, Petkar S. Implantable loop recorders are cost-effective when used to investigate transient loss of consciousness which is either suspected to be arrhythmic or remains unexplained. EP Europace 2012;14:402–09. https://doi.org/10.1093/europace/eur343
