Ivabradine is an I(f)-channel blocker currently used for the treatment of angina and heart failure. Although these channels are known to be found within the sino-atrial node, recent studies have also found localisation within the ventricular myocardium, and there have been reports of ventricular arrhythmia suppression in animal models. We describe an unusual case of significant ventricular ectopy suppression in a patient with non-ischaemic dilated cardiomyopathy. This was accompanied by a significant improvement in percentage pacing from her cardiac resynchronisation device, with corresponding improvement in her functional status. This report suggests, first, that the morbidity and mortality benefit of ivabradine in heart failure may not be solely due to its sino-atrial heart-rate lowering effect, and, second, highlights a potential role for ivabradine in the management of ventricular arrhythmias, which requires further studies to substantiate.
Case history
A 79-year-old woman with a background history of non-ischaemic dilated cardiomyopathy with severe left ventricular (LV) impairment, left-bundle branch block (LBBB) with QRS duration 130–140 ms and LV dyssynchrony, underwent cardiac resynchronisation device implantation after optimisation of her heart failure medication. She continued to remain breathless (New York Heart Association [NYHA] grade III) even after implantation of the device. Device interrogation revealed only 50% pacing due to interference by predominantly unifocal ventricular ectopics (VEs) with VE load of 20% on 24-hour Holter monitoring (figure 1), which did not respond to maximum tolerated dose of beta blockade (carvedilol 12.5 mg twice daily). The patient could not tolerate amiodarone due to hypotension and nausea. Her beta blocker could not be up-titrated due to symptomatic hypotension. While awaiting assessment to consider ablation therapy for the ectopics, she was switched from the beta blocker, to ivabradine 2.5 mg twice daily, up-titrated to 5 mg twice daily, as indicated for heart failure, to achieve a resting heart rate (HR) of around 70 bpm. Her resting HR prior to commencement of ivabradine was 76 bpm. At three-month follow-up, she had significant symptomatic improvement (NYHA grade II). Her repeat 24-hour Holter monitor revealed a significant reduction in VE load from over 20% (figure 1) prior to commencement of ivabradine, to 4% (figure 2). Device interrogation also revealed improvement in ventricular pacing to 70%.
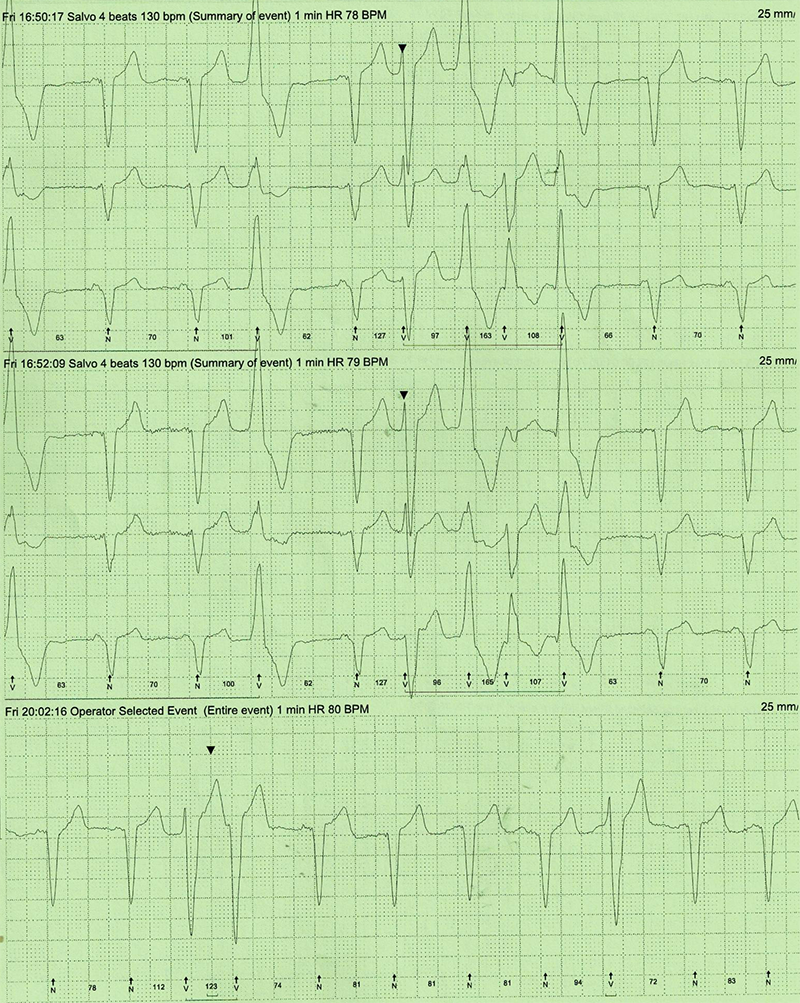
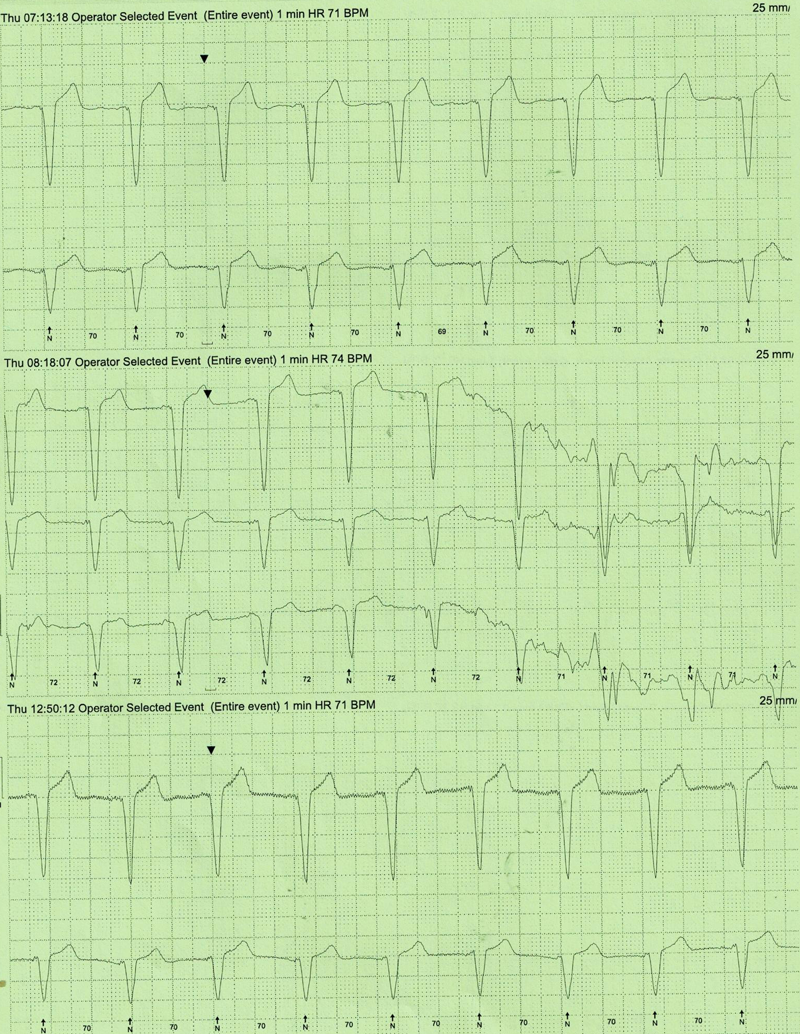
Discussion
Premature ventricular contractions (PVCs) are premature beats arising from ectopic foci within the ventricles. It can be seen in structurally abnormal ventricles, such as in dilated cardiomyopathy. They can be debilitating or life-threatening if there are frequent or prolonged runs, or if sustained ventricular tachycardia develops. In our patient, it significantly interfered with her cardiac resynchronisation device function, and she did not get the initial benefit from this procedure alone.
Ivabradine selectively inhibits the hyperpolarisation-activated cyclic nucleotide-gated (HCN) cation channels which generate the I(f) current (a mixed Na+–K+ inward current). These HCN channels are highly expressed within the sino-atrial node, and suppression by ivabradine lowers the spontaneous pacemaker activity of the sinus node. This reduces the heart rate without changing myocardial contractility or other haemodynamics. Clinically, ivabradine is used as an anti-anginal treatment on the basis of the BEAUTIFUL (Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction) trial.1 More recently, it has also been recommended as a treatment for heart failure with impaired left ventricular systolic function, if heart rate is still above 75 bpm despite beta blockade or if beta blocker intolerant, on the basis of the SHIFT (Systolic Heart failure treatment with the IF inhibitor ivabradine Trial) study.2 Our patient was initiated on ivabradine in accordance with current guidelines for heart failure. This led to significant improvement in functional status, not solely through the heart rate reduction effect, but also through a significant suppression of her PVCs facilitating better cardiac resynchronisation.
Indeed, whether the improved morbidity and mortality in the SHIFT study is attributable solely to the heart rate reduction effect of ivabradine is still a controversy.3 Other drugs that lower the heart rate, such as digoxin for example, have not shown mortality benefit in heart failure. Recent studies have shown localisation of HCN channels within adult ventricular cardiomyocytes, and gain-of-function mutations of these channels in heart failure animal models have been shown to enhance arrhythmogenesis.4,5 Ex vivo studies of ivabradine in canine and human cardiac preparations have shown suppression of ventricular arrhythmias.6 An in vivo rat model demonstrated protection against ventricular arrhythmias in acute myocardial infarction,7 and an in vivo mouse model further showed reduction of infarct size independent of ivabradine’s heart-rate-lowering effect.8 In a rat heart failure model, ivabradine reduced interstitial fibrosis, improved cardiac function, and reduced PVCs by as much as 89%.9 In addition, previous reports have variably stipulated and demonstrated potential anti-arrhythmic properties of ivabradine by:9,10
- LV cardiomyocyte Ca2+ handling
- Prevention of HCN4 up-regulation and inhibition of I(f) in LV cardiomyocytes
- Prevention of action potential duration (APD) dispersion between remote LV wall and infarct burden zone.
These studies not only imply that the benefit of ivabradine in heart failure may lie beyond its sino-atrial heart-rate-lowering effect, but also suggest that there may be a potential role for ivabradine in ventricular arrhythmias. Our striking observations, in this single patient alone, are certainly in keeping with these published reports, but further studies in humans are warranted to explore this potential therapeutic role for ivabradine.
In this patient, we substituted her beta blocker for ivabradine due to intolerance and hypotension, but meta-analyses11 and European heart failure guidelines do suggest benefit of adding ivabradine to beta blockade, as well.
Conflicts of interest
None declared.
Consent
Patient consent for publication was obtained.
Key messages
- Ivabradine may provide protection against ventricular arrhythmia
- Further clinical studies are required to explore this potential of ivabradine
References
1. Fox K, Ford I, Steg PG, Tendera M, Ferrari R; on behalf of the BEAUTIFUL Investigators. Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL). Lancet 2008;372:807–16. https://doi.org/10.1016/S0140-6736(08)61170-8
2. Swedberg K, Komajda M, Böhm M et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 2010;376:875–85. https://doi.org/10.1016/S0140-6736(10)61198-1
3. Pereira-Barretto AC. Cardiac and hemodynamic benefits: mode of action of ivabradine in heart failure. Adv Ther 2015;32:906–19. https://doi.org/10.1007/s12325-015-0257-6
4. Sartiani L, Romanelli MN, Mugelli A, Cerbai E. Updates on HCN channels in the heart: function, dysfunction and pharmacology. Curr Drug Targets 2015;16:868–76. https://doi.org/10.2174/1389450116666150531152047
5. Hermann S, Hofmann F, Stieber J, Ludwig A. HCN channels in the heart: lessons from mouse mutants. Br J Pharmacol 2012;166:501–09. https://doi.org/10.1111/j.1476-5381.2011.01798.x
6. Koncz I, Szél T, Bitay M et al. Electrophysiological effects of ivabradine in dog and human cardiac preparations: potential antiarrhythmic actions. Eur J Pharmacol 2011;668:419–26. https://doi.org/10.1016/j.ejphar.2011.07.025
7. Mackiewicz U, Gerges JY, Chu S et al. Ivabradine protects against ventricular arrhythmias in acute myocardial infarction in the rat. J Cell Physiol 2014;229:813–23. https://doi.org/10.1002/jcp.24507
8. Kleinbongard P, Gedik N, Witting P, Freedman B, Klöcker N, Heusch G. Pleiotropic, heart rate-independent cardioprotection by ivabradine. Br J Pharmacol 2015;172:4380–90. https://doi.org/10.1111/bph.13220
9. Milliez P, Messaoudi S, Nehme J, Rodriguez C, Samuel J-L, Delcayre C. Beneficial effects of delayed ivabradine treatment on cardiac anatomical and electrical remodeling in rat severe chronic heart failure. Am J Physiol Heart Circ Physiol 2009;296:H435–H441. https://doi.org/10.1152/ajpheart.00591.2008
10. Frommeyer G, Weller J, Ellermann C et al. Ivabradine reduces digitalis-induced ventricular arrhythmias. Basic Clin Pharmacol Toxicol 2017;121:526–30. https://doi.org/10.1111/bcpt.12829
11. Komajda M, Böhm M, Borer JS et al. Incremental benefit of drug therapies for chronic heart failure with reduced ejection fraction: a network meta-analysis. Eur J Heart Fail 2018;20:1315–22. https://doi.org/10.1002/ejhf.1234
