Anti-arrhythmic drugs and pulmonary vein isolation (with radiofrequency ablation) are established treatment options in the management of atrial fibrillation. Both methods have their advantages and drawbacks. Atrial fibrillation is the consequence of complex systemic and atrial factors, resulting in atrial remodelling. Emerging treatment strategies that target and reverse atrial remodelling may offer a promising alternative to anti-arrhythmics and ablation.

The question: medicate, or ablate?
To medicate (with anti-arrhythmics), or to ablate: that is the question. In the management of atrial fibrillation (AF), the answer, depending on those questioned and the evidence cited, might be one, the other, both, or neither. Anti-arrhythmic drugs, compared with rate-control drugs, confer no prognostic benefit.1 Furthermore, emerging evidence suggests that ablation confers no mortality benefit over drug therapy.2 This equivocality presents a challenge in clinical practice. Here, consideration is given to each of these standpoints, while highlighting gaps in current knowledge and future directions of AF research and clinical management.
The answer: medicate (with anti-arrhythmics)
Anti-arrhythmic drugs have an important role in the management of AF, notably in the pharmacological cardioversion of AF of recent onset. Compared with electrical cardioversion, pharmacological cardioversion has its benefits (simpler, obviates the need for anaesthesia or fasting) and drawbacks (less effective, acute drug-related side effects). An emerging anti-arrhythmic, vernakalant, has shown superiority compared with established drugs (including amiodarone and flecanide),3 although randomised comparison to electrical cardioversion is required. Long-term rhythm control with anti-arrhythmics also has its merits, often reserved for patients with high AF-related symptom burden despite rate-control, or those who are unsuitable for, or decline, ablation. Benefits in terms of symptom relief must be balanced against risks of extra-cardiac side effects and drug-induced pro-arrhythmias. Despite continued advancements in ablation techniques, certain patients will remain unsuitable for invasive procedures; thus, the future of anti-arrhythmics appears guaranteed, at least provisionally.
The answer: ablate
The development of catheter ablation has transformed the landscape of AF management. Until recently, the prognostic effects of ablation have been debated. The CABANA (Catheter ABlation vs ANtiarrhythmic Drug Therapy in Atrial Fibrillation) trial aimed to assess the safety and efficacy of ablation compared with drugs, with a primary composite end point of mortality, disabling stroke, serious bleeding, and cardiac arrest.2 Dr Douglas Packer recently announced the results of this randomised, single-blinded trial of 2,204 patients at the 39th Heart Rhythm Society’s Scientific Sessions. Compared with drug therapy, ablation did not significantly reduce the primary end point, although high crossover rates in both arms may have affected these results. The trial findings are summarised in table 1, alongside awaited trials4 and unanswered questions in AF management. Importantly, CABANA was not designed to detect symptomatic response to ablation. To do so, a randomised, double-blinded, placebo-controlled trial is necessary, to distinguish symptomatic improvement truly attributable to ablation from the placebo effect of an invasive procedure.5
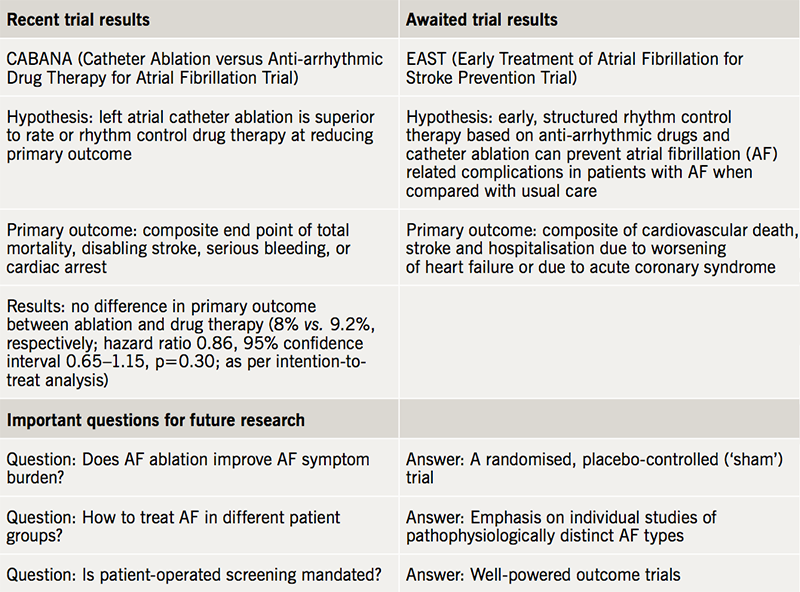
One AF subgroup in which the prognostic benefits of ablation are known is patients with concomitant heart failure with reduced ejection fraction: ablation results in significantly lower mortality and hospitalisation for worsening heart failure, compared with medical therapy.6 Despite a paucity of randomised data, ablation is also often performed in symptomatic younger patients with paroxysmal AF and no apparent comorbidities, as first-line therapy or after unsuccessful treatment with anti-arrhythmics. Demonstrating improved long-term outcomes in this group is challenging and necessitates large trials with considerable follow-up periods, owing to low AF-related event rates in this population. Ablation in asymptomatic patients is equally contentious.7
Regardless, procedure-related risks must be balanced against the risks of alternative treatment strategies and those of remaining in AF. Theoretically, development of non-invasive ablation strategies, including stereotactic pulmonary vein radioablation, could circumvent certain of these risks, with pre-clinical models providing promising results.8
The answer: both
Categorising ablation as superior to anti-arrhythmics (or vice versa) ignores the subtleties of individual patient characteristics, concerns and expectations. In reality, both strategies play complementary roles. Concomitant short-term anti-arrhythmic therapy may be advantageous in the pre- and post-ablation period,9 and long-term anti-arrhythmic therapy may be used for symptomatic benefit following unsuccessful ablations. Over time, patient preferences evolve, with hybrid drug-ablation strategies common.
The answer: neither (look further upstream)
For the foreseeable future, anti-arrhythmic drugs and ablation will likely maintain their roles in the management of AF. Arguably, the long-term future of the condition lies elsewhere. The complexity of AF resides in the combination of systemic and local atrial factors linked to its pathophysiology. AF is frequently (although not invariably) associated with systemic disease and risk factors, including obesity, sleep apnoea, hypertension, smoking, excess exercise or alcohol; AF may represent the end-organ manifestation of these conditions.10 At an atrial level, AF is characterised by atrial remodelling, as a result of electrical, metabolic, autonomic, and structural changes (fibrosis, inflammation, hypertrophy, and oxidative stress).10 Rhythm control strategies attempt to manage the consequences of these processes, without preventing or modulating these factors per se. An increasing body of evidence supports the concept of ‘upstream’ therapy10 in the management of AF (figure 1). This refers to the targeting of systemic and atrial processes, in order to pre-empt or modify the atrial substrate.
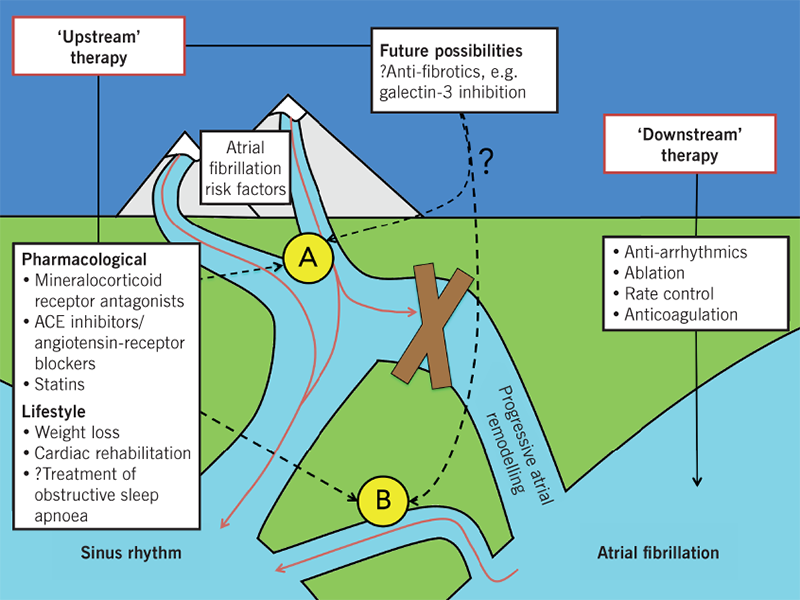
Observational studies (prone to bias and confounding) provide an insight into the role of lifestyle modification in AF: long-term sustained weight loss is associated with significant reduction in AF burden and maintenance of sinus rhythm,11 and improvement in cardiorespiratory fitness augments the beneficial effects of weight loss.12 These findings are strengthened by a recent randomised trial13 in which patients with persistent, early, and symptomatic AF were assigned to ‘conventional’ therapy with anti-arrhythmics or ‘targeted’ therapy of underlying conditions (including hypertension, obesity, hypercholesterolaemia and heart failure) with pharmacological interventions and cardiac rehabilitation. One year following electrical cardioversion, sinus rhythm was significantly more likely in the intervention group compared with the conventional group.13 Whether this translates into prognostic outcomes is unclear. Certainly, these findings are not applicable to all, and targeting obesity and fitness is more challenging for clinicians (and patients) than treatment with medications or procedures. Implementing meaningful lifestyle interventions requires specialised and well-funded services that educate and empower patients, in addition to the backing of physicians, particularly electrophysiologists. With time, anti-arrhythmics and ablation could become adjunctive methods to ‘upstream’ therapy, used to temporarily reduce symptom burden, allowing patients the time to implement meaningful lifestyle changes, and physicians the time to target underlying conditions.
Finally, targeting fibrotic pathways within the atrial myocardium may form an integral part of future ‘upstream’ therapies. In patients with AF, raised galectin-3 levels (a biomarker of fibrosis) are associated with increased disease severity, decreased treatment efficacy, and poorer outcomes.14 In a sheep-model, pharmacological galectin-3 inhibition reduces AF inducibility and AF burden, and increases AF termination.14 Greater understanding of these pathways may facilitate the development of more effective therapeutic ‘upstream’ strategies.
Moving forward
Medicate, or ablate: that is the question. But is it the correct question? Undeniably, both strategies have their place in specific cohorts, with patient selection crucial. Often, however, neither will succeed if ‘upstream’ factors are not addressed. AF is commonly the manifestation of systemic disease, including obesity and hypertension. Treating and preventing AF implies managing the complex electrical, metabolic, autonomic, and structural changes occurring in the atria. In addition to novel therapeutics that modulate these changes, the future of AF lies in holistic lifestyle modification. The CABANA trial results provide some clarifications to the rhythm control debate,2 while simultaneously generating further questions. Nevertheless, to truly appreciate the effects of ablation on AF symptom burden, a randomised and placebo-controlled trial (or so-called ‘sham’ trial) is necessary, which despite logistical challenges and ethical trepidations is feasible.5 Ultimately, rather than ‘medicate or ablate’, the future might be ‘communicate, educate, and modulate’, alongside current treatment options.
Conflicts of interest
None declared.
Key messages
- Despite their side effect profile, anti-arrhythmic drugs are important in the management of symptomatic patients with atrial fibrillation (AF), particularly those unsuitable for ablation
- Ablation has prognostic benefits in patients with concomitant heart failure with reduced ejection fraction. The prognostic benefit in a wider cohort of patients with AF is less clear. Symptomatic response to ablation can only be assessed by a randomised, double-blinded, placebo-controlled trial
- The future of AF management lies in holistic lifestyle modification and novel therapeutics that target atrial remodelling
References
1. Wyse DG, Waldo AL, DiMarco JP et al.; AFFIRM Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002;347:1825–33. https://doi.org/10.1056/NEJMoa021328
2. Packer DL, Mark DB, Robb RA et al. Catheter ablation versus antiarrhythmic drug therapy for atrial fibrillation (CABANA) trial: study rationale and design. Am Heart J 2018;199:192–9. https://doi.org/10.1016/j.ahj.2018.02.015
3. Capucci A, Cipolletta L, Guerra F, Giannini I. Emerging pharmacotherapies for the treatment of atrial fibrillation. Expert Opin Emerg Drugs 2018;23:25–36. https://doi.org/10.1080/14728214.2018.1446941
4. Kirchhof P, Breithardt G, Camm AJ et al. Improving outcomes in patients with atrial fibrillation: rationale and design of the early treatment of atrial fibrillation for stroke prevention trial. Am Heart J 2013;166:442–8. https://doi.org/10.1016/j.ahj.2013.05.015
5. Al-Lamee R, Thompson D, Dehbi HM et al. Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomised controlled trial. Lancet 2018;391:31–40. https://doi.org/10.1016/S0140-6736(17)32714-9
6. Marrouche NF, Brachmann J, Andresen D et al.; CASTLE-AF Investigators. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 2018;378:417–27. https://doi.org/10.1056/NEJMoa1707855
7. Kalman JM, Sanders P, Rosso R et al. Should we perform catheter ablation for asymptomatic atrial fibrillation? Circulation 2017;136:490–9. https://doi.org/10.1161/CIRCULATIONAHA.116.024926
8. Zei PC, Wong D, Gardner E et al. Safety and efficacy of stereotactic radioablation targeting pulmonary vein tissues in an experimental model. Heart Rhythm 2018;15:1420–7. https://doi.org/10.1016/j.hrthm.2018.04.015
9. Darkner S, Chen X, Hansen J et al. Recurrence of arrhythmia following short-term oral AMIOdarone after CATheter ablation for atrial fibrillation: a double-blind, randomized, placebo-controlled study (AMIO-CAT trial). Eur Heart J 2014;35:3356–64. https://doi.org/10.1093/eurheartj/ehu354
10. Savelieva I, Kakouros N, Kourliouros A et al. Upstream therapies for management of atrial fibrillation: review of clinical evidence and implications for European Society of Cardiology guidelines. Part I: primary prevention. Europace 2011;13:308–28. https://doi.org/10.1093/europace/eur002
11. Pathak RK, Middeldorp ME, Meredith M et al. Long-term effect of goal-directed weight management in an atrial fibrillation cohort: a long-term follow-up study (LEGACY). J Am Coll Cardiol 2015;65:2159–69. https://doi.org/10.1016/j.jacc.2015.03.002
12. Pathak RK, Elliott A, Middeldorp ME et al. Impact of CARDIOrespiratory FITness on arrhythmia recurrence in obese individuals with atrial fibrillation: the CARDIO-FIT study. J Am Coll Cardiol 2015;66:985–96. https://doi.org/10.1016/j.jacc.2015.06.488
13. Rienstra M, Hobbelt AH, Alings M et al. Targeted therapy of underlying conditions improves sinus rhythm maintenance in patients with persistent atrial fibrillation: results of the RACE 3 trial. Eur Heart J 2018;39:2987–96. https://doi.org/10.1093/eurheartj/ehx739
14. Clementy N, Piver E, Bisson A et al. Galectin-3 in atrial fibrillation: mechanisms and therapeutic implications. Int J Mol Sci 2018;19:E976. https://doi.org/10.3390/ijms19040976
