Absence of the pericardium is a rare defect that can be both congenital and acquired. Defects occur through abnormal development of the pleuro-pericardial membranes, which should fuse at the midline and separate the pericardial and pleural cavities.1 Congenital incidence is thought to be less than one in 10,000,2 however, prevalence is uncertain due to the incidental findings of many diagnoses. With increasing use of cardiac magnetic resonance imaging (CMR) and cardiac computed tomography (CT), diagnosis of pericardial absence is becoming more frequent, however, little is known about the long-term management of these patients.
Case
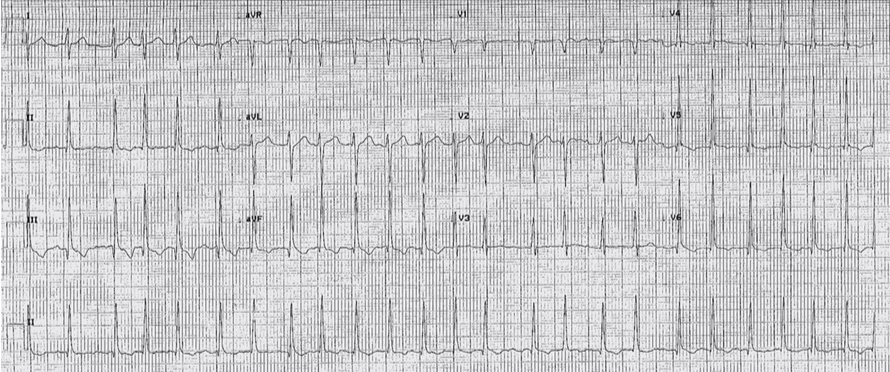
We present a 31-year-old professional golfer with no significant cardiac medical history, who presented to Aberdeen Royal Infirmary in the Summer of 2015. He described palpitations after drinking alcohol the night before. After further investigation he was found to be in atrial fibrillation (AF) with fast ventricular rate (figure 1), and underwent medical cardioversion with bisoprolol and flecainide. His blood results were normal and he was discharged with an outpatient echocardiogram follow-up.
Transthoracic echocardiogram in August 2015 revealed good left ventricular systolic function and a non-dilated left ventricle, however, it did reveal non-standard views throughout the entire scan. Echocardiogram was unable to clearly identify the right ventricular free wall and also revealed a bright bulbous area in the mid-right ventricular free wall in the modified apical four-chamber view. A CMR was recommended to assess the right ventricular size and function.
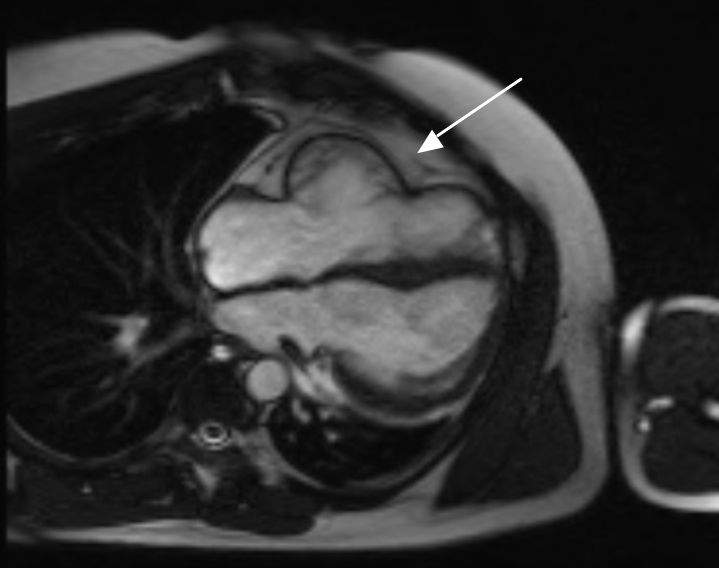
The CMR revealed postero-lateral positioning of the left ventricle with an unusual cardiac axis showing clockwise rotation of the apex, suggestive of a right-sided partial absence of the pericardium (figure 2). A reduction in left lung volume was a consequence of the altered cardiac position. There was a bulbous appearance of the right ventricular free wall, with normal right ventricular indexed volumes and good systolic function. It was thought the bulbous appearance of the right ventricular free wall alone was not sufficient for a CMR diagnosis of arrhythmogenic right ventricular cardiomyopathy (ARVC) and a cardiac CT was recommended for further information. Mild tricuspid regurgitation was also noted.
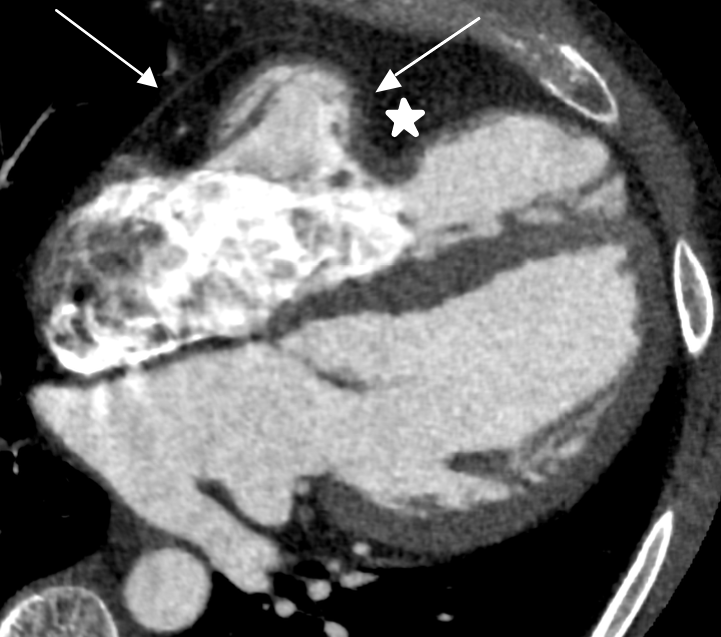
As the initial CMR raised the question of possible partial absence of the pericardium, a CT was advised for confirmation. Figure 3 demonstrates an ECG-gated cardiac CT scan in mid-diastole. The scan successfully identified the distortion caused by the pericardial abnormality to the right ventricular free wall and specifically shows the termination of the pericardium. Thus, indicating the use of CT as the gold-standard investigation for this case.
Discussion
Congenital absence of the pericardium is an uncommon defect that is often asymptomatic, however, it can present with life-threatening complications.3 Depending on the size and location of the defect, it can also mimic other cardiac conditions such as ARVC, cardiac neoplasms and structural defects.2 Our patient was unusual in that he was found to have a right-sided partial defect, which usually represents 17% of cases, as opposed to left-sided defects at 70%.1 Right-sided defects are generally not seen as often, as the right duct of Cuvier normally persists as the superior vena cava, which facilitates closure of the right pleuro-pericardial membranes.2 Complications arise in partial defects via herniation and strangulation of the cardiac structures and compression of the coronary arteries.4 In such cases, surgical correction would be warranted. Similarly, it is likely that atrial compression, secondary to the pericardial ‘window’, has led to the development of AF in this patient. Alternatively, elevated filling pressures leading to atrial constraint could also have contributed to the arrhythmia.
Due to the variation in initial presentations of this cohort of patients, a number of different investigatory methods need to be employed to arrive at a conclusive diagnosis. Chest radiograph has been found to be useful in identifying left-sided defects, with a ‘tongue’ of lung tissue between the aorta and the main pulmonary artery seen and leftward deviation of the cardiac silhouette, which results in loss of the right heart border due to the superimposition onto the spine.5 Alternatively, with right-sided defects, a relative lucency can be seen due to the interposed lung between the right pulmonary artery and the aorta, or between the right inferior cardiac border and right hemidiaphragm. Disruption in the cardiac silhouette can also be seen with herniation of cardiovascular or pulmonary structures through the defect.
Cardiac CT or CMR are needed to confirm the diagnosis. CMR has been widely used due to the ability to identify the location and extent of the pericardial absence,6 as well as using functional characteristics, such as excessive cardiac mobility and large difference in total heart volume in the end systolic and diastolic phases.7 As with this case, cardiac CT proved to be diagnostic due to the enhanced spatial resolution compared with CMR, superior delineation and the ability to visualise calcification, and should be considered as gold standard when visualising pericardial anatomy.
As such, our patient was presented at our local multi-disciplinary team (MDT) meeting following his third CMR, as part of routine surveillance. There had been no changes in appearance of the heart structure since diagnosis in 2016 and the patient had remained asymptomatic with no further episodes of AF. Which presents the question; how much surveillance should routinely be offered to patients with partial absence of the pericardium? Little literature offers a standard procedure for such cases. The local MDT outcome was to continue to monitor with yearly CMRs in the absence of new symptoms.
There is a paucity of literature to suggest a standard practice for management and surveillance of this problem, however, estimation of risk is essential to identify those with a higher chance of complication. It is thought that smaller defects in partial absence, represent a higher risk for life-threatening complications and, therefore, those patients should be offered surgery, even if the patient is asymptomatic. Larger partial defects and complete left- or right-sided defects have lower or no risk, respectively, of herniation and strangulation of the cardiac tissues and, therefore, surveillance may be a more prudent approach.6
In conclusion, with improved imaging, the diagnosis of congenital absence of the pericardium is becoming easier, however, the long-term surveillance of this cohort of patients is still lacking in structure and evidence. As there can be life-threatening complications for a potentially young demographic, it is important to consider a more structured approach, and with CMR and CT this may now be possible. Further research needs to be undertaken using these techniques, to indicate the size of defect in the context of the symptomatic or asymptomatic patient and their subsequent morbidity and mortality.
Key messages
- Congenital absence of the pericardium can present with non-specific cardiac signs and a high index of suspicion is required to identify the cause of the problem
- Cardiac computed tomography (CT) has been shown, in this case, to be the gold standard for the successful diagnosis of the partial absence of the pericardium, due to superior identification of the pericardial anatomy
- Congenital absence of the pericardium can result in life-threatening complications, however, there is currently little in the way of standard practice for the management and long-term surveillance of these patients
- Further development of risk stratification, according to defect size needs to be the focus of future research to aid management decisions
Conflicts of interest
None declared.
Consent
The patient provided consent for publication.
References
1. Kim HJ, Cho YS, Cho GY, Choi SI. Congenital absence of the pericardium. J Cardiovasc Ultrasound 2014;22:36–9. https://doi.org/10.4250/jcu.2014.22.1.36
2. Shah AB, Kronzon I. Congenital defects of the pericardium: a review. Eur Heart J Cardiovasc Imaging 2015;16:821–7. https://doi.org/10.1093/ehjci/jev119
3. Wan-Koo C, Newburg A. Congenital absence of the right pericardium: embryology and imaging. J Clin Imaging Sci 2015;5:12. https://doi.org/10.4103/2156-7514.152338
4. Foo JS, Koh CH, Sahlén A, Tang HC, Lim CP. Congenital absence of pericardium: a mimic of arrhythmogenic right ventricular cardiomyopathy. Case Rep Med 2018;2018:4297280. https://doi.org/10.1155/2018/4297280
5. Gatzoulis MA, Munk M-D, Merchant M, Van Arsdell GS, McCrindle BW, Webb GD. Isolated congenital absence of the pericardium: clinical presentation, diagnosis and management. Ann Thorac Surg 2000;69:1209–15. https://doi.org/10.1016/S0003-4975(99)01552-0
6. Brulotte S, Roy L, Larose E. Congenital absence of the pericardium presenting as acute myocardial necrosis. Can J Cardiol 2007;23:909–12. https://doi.org/10.1016/S0828-282X(07)70851-6
7. Psychidis-Papakyritsis P, De Roos A, Kroft LJM. Functional MRI of congenital absence of the pericardium. AJR Am J Roentgenol 2007;189:W312–W314. https://doi.org/10.2214/AJR.05.1655
