This short review of cardiac tumours presents a case that clearly demonstrates the manifestation of embolic and cardiac symptoms of an intracardiac mass. Acute onset and rapid progression of a neoplastic process in the heart leading to arrhythmia, cardiac conduction disorders and heart failure combined with highly mobile fragments of tumour, which can cause emboli in cerebral vessels, are characteristic signs of an intracardiac mass. Early diagnosis and immediate treatment may improve the long-term prognosis, but overall the prognosis is poor. Cardiac tumours present to the cardiologist when the patient presents with cardiac symptoms, and the neurologist when there are cerebral symptoms. Most cardiac masses are not amenable to percutaneous biopsy; therefore, definitive diagnosis often awaits surgical excision.
Introduction
Cardiac tumours are a heterogeneous group of neoplasia growing from the heart tissue or the pericardium. Primary tumours of the heart are rare, they only constitute 0.5% of all tumours. Of those, 75% are benign and 25% malignant.1 Most malignant tumours are sarcomas. Recognition of a primary heart neoplasia is extremely difficult due to the variable symptoms. Malignant tumours are usually rapidly progressive, while benign tumours, such as myxomas, may exhibit symptoms over many years. Symptoms are various, depending on growth, size, location, possible cardiac obstruction and disorders of the cardiac conduction system.1
The fourth edition of the World Health Organization (WHO) Classification of Tumours of the Lung, Pleura, Thymus and Heart presents a wide classification of cardiac tumours, which are divided into benign, and malignant tumours.2
Diagnosis
Echocardiography is the most effective, non-invasive method to diagnose a cardiac tumour. The sensitivity is 93% for transthoracic echo (TTE) to 97% for transoesophageal echo (TOE) in cases of heart neoplasms. Location of the tumour influences the preferred method; TOE is more often used to visualise atria, and TTE is used to visualise ventricles. These methods allow determination of accurate size, location and mobility of the tumour, as well as its influence on other anatomic structures, and to define myocardial contractility, intracardiac haemodynamics and the functional state of the heart valves.3 Use of magnetic resonance imaging (MRI) has also improved diagnosis.
The characteristics of a malignant tumour are an acute onset and fast progression of symptoms. Clinical signs of heart tumours can be classified in four groups.
- Systemic manifestations. Primary tumours of the heart, particularly myxomas, often cause general symptoms, such as fever, malaise, chills, weakness and weight loss. General signs may confuse diagnosis. In addition, some symptoms like myalgia, arthralgia, muscle fatigue and Raynaud’s syndrome can imitate vasculitis or collagen-vascular diseases. Blood tests might show leucocytosis, polycythaemia, anaemia, thrombocytosis or, on the other hand, thrombocytopenia, as well as hypergammaglobulinaemia with high erythrocyte sedimentation rate (ESR).
- Embolism. Malignant primary tumours of the heart are often accompanied by embolism. This may be due to a crumbly and, sometimes, necrotised inner surface of the tumour. Nearly every organ can be affected by a thrombus released from the surface of the sarcoma. This may lead to ischaemia, infarct or even aneurysm in the future.
- Cardiac impact. Sarcomas can impact the mechanics of the myocardium, cardiac valves or the coronary system, as well as electrical conduction. Cardiac sarcomas that occur on the pericardium can also cause increased fluid collection in the pericardial cavity, which then affects the pumping ability. Most of intramural primary heart tumours are asymptomatic, especially when they are small. Larger ones that are located close to large conduction pathways of the heart, can constrict them and lead to numerous arrhythmias and conduction disorders, including complete heart block or even asystole. Also, large intramural tumours can squeeze cardiac cavities and cause ventricular outflow tract obstruction or mitral regurgitation.
- Metastases. Most malignant tumours of the heart are diagnosed at an advanced stage, when systemic dissemination of malignant cells is already present. In some cases, secondary symptoms caused by metastases may appear earlier than primary cardiac symptoms.4,5
Case presentation
In August 2016, a 64-year-old woman presented with complaints of paroxysmal atrial fibrillation. In September 2016 and February 2017, the patient went through intracardiac electrophysiology studies and radiofrequency catheter ablation, antral isolation of pulmonary veins ostia and of cavo-tricuspid isthmus. In August 2017, the first onset of atrial fibrillation after the ablation was registered. Echocardiography showed dilatation of the left atrium with a regular size of other chambers. The contractility of the left ventricle was not affected. There was no ventricular or atrial septal defect detected. Additionally, aortic, mitral and tricuspid insufficiencies grade I were diagnosed.
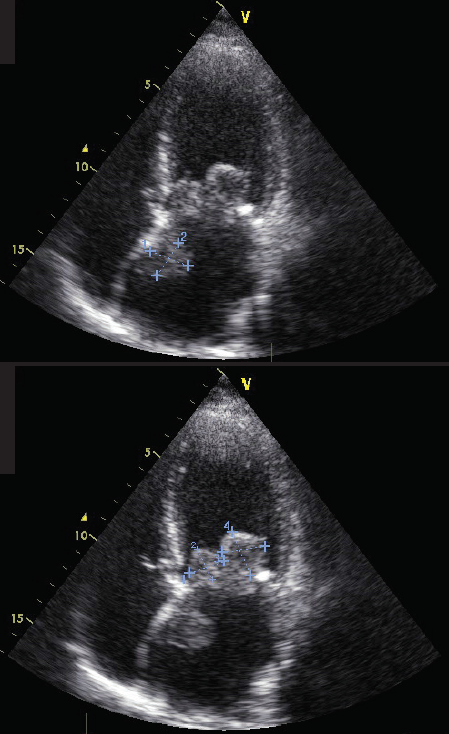
cusps of the mitral valve, and one originating from the interatrial septum (apical 4-chamber probe position)
In September 2017, the patient presented again with episodes of irregular heartbeat, cough, dyspnoea and weakness. In the same month, the patient described a syncope with a memory loss. A new echocardiogram (figure 1) confirmed massive round lumps on both leaflets of the mitral valve. Those lumps were moving together with the leaflets (‘cusps’) and prolapsing into the left ventricle in the diastole, narrowing the opening of the mitral valve. The mass on the anterior cusp was sized 21 × 29 mm, and the one on the posterior cusp sized 33 × 23 mm. In addition, echocardiography showed other masses on the lump of the posterior cusp, sizing to 9 × 5 mm. A third mass in the left atrium was detected fixed on the atrial septum, size 22 × 24 mm. Other echo parameters were: Vmax 2.4 m/s, pressure gradient (PG) peak 23 mmHg, mean PG 12 mmHg, surface area of the mitral valve 1.56 cm2, mitral insufficiencies I–II grade. She was referred for surgery. In October 2017, the suspicious masses in the left atrium were removed, and the diseased mitral valve was replaced by a mechanical prosthetic (Medtronic-29) under artificial circulation and combined chemical/hypothermic cardioplegia. Postoperative echo showed the following parameters: PG peak 9 mmHg, mean PG 3 mmHg. Also, the correct position and the functionality of the prosthetic were ensured. No fluid was detected in the pericardium or pleural cavity.
The histological examination confirmed an undifferentiated pleomorphic sarcoma with areas of necrosis and numerous cells undergoing mitosis. Thus, the diagnosis “undifferentiated pleomorphic sarcoma of the left atrium endocardium” was made. These are believed to originate in pluripotential stem cells.2 After the relevant surgery, the patient went through the necessary chemotherapy. She has no current clinical disorders, and her state is stable. She is regularly examined by cardiologists and oncologists, and a recent echocardiogram showed good functioning of the prosthesis.
Discussion
The presented case clearly demonstrates the manifestation of embolic and cardiac symptoms of an intracardiac mass. Acute onset and rapid progression of a neoplastic process, highly mobile fragments of masses (which cause emboli in cerebral vessels), arrhythmia, cardiac conduction disorders and heart failure are characteristic signs of a malignant intracardiac process. Early diagnosis and immediate treatment may improve the long-term prognosis and the quality of life, despite the serious pathology. We are reporting this case for educational and clinical purposes, due to the rare case of undifferentiated pleomorphic sarcoma (because myxoma is the most common primary cardiac tumour, found in the left atrium in 75% of cases).
Key messages
- Cardiac tumours are comparatively rare
- A short review of literature on cardiac tumours is provided
- An undifferentiated pleomorphic sarcoma of the left atrium endocardium is described
Conflicts of interest
None declared.
Funding
None.
Consent
Patient consent was obtained.
References
1. Kranin DL. Cardiac masses due to tumors (diagnosis, clinical manifestations and surgical treatment). Kazan Medical Journal 2014;6:806–10. Available from: https://kazanmedjournal.ru/kazanmedj/article/view/1985
2. Burke A, Tavora F. The 2015 WHO classification of tumors of the heart and pericardium. J Thorac Oncol 2016;11:441–52. https://doi.org/10.1016/j.jtho.2015.11.009
3. Akhunova SY. Echocardiographic diagnosis of cardiac space-occupying lesions. Practical Medicine 2017;2:28–33. Available from: http://en.pmarchive.ru/echocardiographic-diagnosis-of-cardiac-space-occupying-lesions/
4. Mikhoparova OY. Cardiac tumors in clinical practice. Vestnik Sovremennoi Klinicheskoi Mediciny 2017;4:80–6. https://doi.org/10.20969/VSKM.2017.10(4).80-86
5. Andrushchuk VV. Surgery of malignant heart tumors. Eurasian Heart Journal 2016;3:178.
Additional reading
Patel J, Sheppard MN. Pathological study of primary cardiac and pericardial tumours in a specialist UK Centre: surgical and autopsy series. Cardiovasc Pathol 2010;19:343–52. https://doi.org/10.1016/j.carpath.2009.07.005
Hegyi L, Thway K, Fisher C, Sheppard MN. Primary cardiac sarcomas may develop from resident or bone marrow-derived mesenchymal stem cells: use of immunohistochemistry including CD44 and octamer binding protein 3/4. Histopathology 2012;61:966–73. https://doi.org/10.1111/j.1365-2559.2012.04299.x
