Various cardiac disorders seen in general and acute medicine have dermatological manifestations that may provide critical clues to the underlying disease. This review will discuss the important dermatological signs seen in cardiac conditions. We believe greater interdisciplinary liaison will improve our understanding of the link between the dermatological and cardiovascular systems and the underlying disease processes.
Introduction
Table 1. Summary of general dermatological signs
| Dermatological sign | Cardiac disorder |
|---|---|
| Xanthomata | Hyperlipidaemia |
| Acanthosis nigricans | Obesity, diabetes, hyperinsulinaemia, metabolic syndrome |
| Male-pattern baldness | Coronary heart disease |
| Premature hair greying | Coronary heart disease, hyperlipidaemia |
| Earlobe crease | Coronary heart disease |
| Livedo reticularis | Cholesterol embolisation syndrome, anti-phospholipid syndrome/systemic lupus erythematosus, endocarditis, rheumatic fever, diabetes |
| Cyanosis | Congenital heart disease, heart failure |
| Clubbing | Congenital heart disease, endocarditis, cardiac myxoma |
Cardiac conditions have signs and symptoms affecting several organs. Notably, several common and important cardiac conditions may have dermatological manifestations, which may point to and aid in diagnosis, as well as guide further investigations and management. Here, we will discuss dermatological manifestations of important cardiac conditions starting with some general dermatological signs and covering specific pertinent conditions.
General dermatological signs in cardiac disorders
These general signs are summarised in table 1 along with their associated cardiac disorders.
Xanthomata
Xanthomata are yellow cholesterol-rich deposits that accumulate in foam cells, which can be seen anywhere in the body. The most common form is called xanthelasma palpebrarum (XP), where deposits are seen on the eyelid (figure 1). XP is an independent risk factor for ischaemic heart disease (IHD). Its prevalence in the Western population has been estimated as between 0.56 and 1.5%.1 Roughly half of patients with XP have hyperlipidaemia, but other causes include cirrhosis, hypothyroidism, nephrotic syndrome and systemic disorders, such as Erdheim-Chester disease. Age of onset varies, but if found in those under 40 years, familial hyperlipidaemia should be suspected. Histologically, XP are formed from lipid-laden histiocytes, primarily located within the upper reticular dermis or perivascular and periadnexal spaces. Although treatment can be offered for cosmesis, recurrence is common.

Xanthomata may be seen in other areas in addition to the eyelids. They may extend to the connective tissue layer in the skin, tendons, fascia and sometimes periosteum. Eruptive xanthoma occurs due to the sudden eruption of fatty deposits, often on the buttocks, limbs or back. They are associated with severe hypertriglyceridaemia and chylomicronaemic syndrome. Chylomicronaemic syndrome is a rare inherited disorder leading to elevated chylomicron levels in plasma, leading individuals susceptible to complications such as acute pancreatitis. Tuberous xanthomata are firm, painless nodules that develop around pressure points. They are seen in familial hypercholesterolaemia or dysbetalipoproteinaemia. Tendinous xanthomata are a form of slower growing xanthoma associated with the underlying tendons, tendon attachments, ligaments, fascia and periosteum. They are often seen in the hands, feet and Achilles tendon and are associated with higher levels of hypercholesterolaemia. Planar xanthomata are flat papular lesions occurring anywhere in the body. When seen in the palmar creases, they may be referred to xanthoma striatum palmare, which are particularly associated with type III hyperlipoproteinaemia where there is accumulation of beta-very-low-density lipoprotein (beta-VLDL). Xanthoma diffusum planum may present as yellow-red plaques in the face, neck and skin folds. Interestingly, it is not normally associated with lipid disorders, but may be related to lymphoproliferative or myeloproliferative disease. Lastly, xanthoma disseminatum presents with several yellow-red-brown papules or nodules, which are often seen in a symmetrical distribution in the trunk and face. It is, again, not usually associated with lipid disorders, but instead is often a self-limiting, rare form of histiocytosis. However, internal organ involvement may be seen and meningeal involvement leads to diabetes insipidus in around 40% of patients.
Acanthosis nigricans (AN)

AN (figure 2) is characterised by hyperkeratotic and hyperpigmented velvety lesions occurring mainly in skin folds such as the axilla, groin and back of neck. It is associated with several different conditions including obesity, hyperinsulinaemia, Cushing’s syndrome, polycystic ovary syndrome (PCOS), diabetes, metabolic syndrome and malignancy. It may also be benign, in this case more often in dark-skinned individuals. Diagnostic work should aim to identify and correct the underlying disease process. Preliminary investigations may include a diabetes screen, endocrine bloods for PCOS and Cushing’s, and an ultrasound of the ovaries if PCOS is suspected. If malignant AN is suspected, prompt workup to identify the source of malignancy should be undertaken, as malignancies in the presence of AN are often aggressive.
Male-pattern baldness and premature greying
Male-pattern baldness (MPB) is the most common cause of alopecia in men: 80% of men are affected by the age of 80 years. However, MPB may present much earlier. A recent meta-analysis suggests vertex baldness rather than frontal baldness is associated with an increased risk of coronary heart disease among men of all ages (p=0.008) and younger men (p=0.006).2 The reason for this is unclear, but may be related to hypertension, smoking status, metabolic syndrome and insulin resistance, which all promote atherosclerosis. The association is not clear cut, as a Turkish study showed no relation between the presence, severity or age of onset of MPB and coronary artery disease (CAD) in multi-variate analysis.3
Greying of hair is part of the natural ageing process. However, premature ageing has been seen to be indicative of an increased risk for several disease processes, including CAD, independent to age and other cardiovascular risk factors.4 The exact mechanism for this relationship is not known, but it is thought that there may be overlap in the underlying mechanisms for atherosclerosis and premature hair greying.4 Hair greying is also associated with other risk factors, such as smoking, age, hyperlipidaemia, creatinine levels and family history.
Frank’s sign
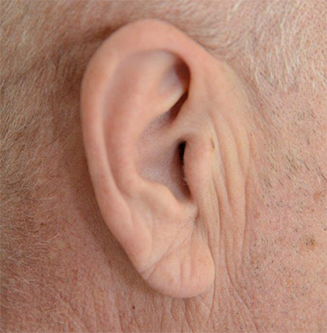
A diagonal earlobe crease (figure 3), first characterised by Frank in 1973,5 has been associated with cardiovascular disease. An early study suggested the sign was independently associated with obstructive CAD, although also related to ageing.6 Indeed, symptomatic patients were more likely to have extensive disease if Frank’s sign was present. A meta-analysis of over 30,000 patients suggested that patients with Frank’s sign were 3.3 times more likely to have CAD.7
However, this association does remain controversial. A study in type 2 diabetics, showed Frank’s sign was not a significant predictor of CAD in multi-variate analysis.8 Indeed, others have suggested that factors such as age, ethnicity and earlobe shape may have an important impact on the presence of earlobe creasing.9
The exact pathogenesis of this sign is debated. However, biopsy samples from the earlobe crease show loss of elastic and elastin fibres, in keeping with microvascular disease.
Livedo reticularis

Livedo reticularis (LR) (figure 4) refers to a mottled net-like discolouration of the skin. Various forms exist and the phenomenon may be primary or secondary. Livedo racemosa describes a more generalised persistent form, which, although classically described in Sneddon’s syndrome, is also seen in other conditions, such as antiphospholipid syndrome (APS), systemic lupus erythematosus (SLE) and polyarteritis nodosa. A physiological form of LR, also referred to as cutis marmorata, is often seen due to cold exposure in young women. Histopathology varies depending on the cause, and, indeed, in primary and physiological forms, no abnormalities may be detected.
The differential for LR is wide, but may include haematological conditions, autoimmune/vasculitis/connective tissue disorders, malignancy, infections and neurological disease. In relevance to cardiac conditions, particular associations with cholesterol embolisation syndrome, APS, SLE, endocarditis, rheumatic fever and diabetes have been described. Preliminary investigations would depend upon the presentation, but may include a vasculitis screen, inflammatory markers, clotting/coagulation screen and a lipid profile. If the diagnosis is in doubt, biopsies may be undertaken.
Other general signs include clubbing and cyanosis, which both have causes from multiple systems.
Specific cardiac conditions
Table 2. Dermatological signs associated with specific cardiac conditions
| Cardiac condition | Dermatological signs |
|---|---|
| Infective endocarditis | Clubbing, Osler’s nodes, Janeway lesions, purpura, splinter haemorrhages |
| Rheumatic heart disease | Erythema marginatum, subcutaneous nodules, skin ulcers |
| Cardiac myxoma | Cyanosis, petechiae, pruritic lesions, livedo reticularis, vasculitis, hyperpigmentation, Raynaud’s |
| Cardiac sarcoma | Cutaneous metastasis |
| LEOPARD syndrome | Lentigines, hypopigmented macules, café-au-lait spots |
| Kawasaki disease | Oropharyngeal/palmar erythema, rash, cracked lips, strawberry tongue, Beau’s lines, hand/feet oedema, periungual desquamation |
| Cardiac transplantation | Skin cancer, skin infections, hypertrichosis, steroid-induced manifestations, malakoplakia, seborrhoeic dermatitis, keloid, Koebnerisation |
| Cardiac devices | Keloid, Koebnerisation, contact dermatitis |
Several specific cardiac conditions have numerous associated dermatological signs, which are summarised in table 2.
Infective endocarditis
Infective endocarditis (IE) is the infection of heart valves and endocardial wall. This is a potentially deadly infection with inpatient mortality approaching 20%, mostly due to sepsis, stroke and heart failure. The organisms may gain access to the cardiovascular system through a variety of routes. These include the respiratory, gastrointestinal and genitourinary tracts, as well as via the skin in around 20% of cases.
Dermatological manifestations may include clubbing, Osler’s nodes, Janeway lesions, purpura and splinter haemorrhages. Osler’s nodes are typically painful purple nodes, often on the fingertips, which are associated with vasculitis. Janeway lesions are painless and often found on the palms or soles and may last for weeks. The prevalence of lesions such as Osler’s nodes and Janeway lesions is estimated to be 5–15%.10 However, as the dermatological manifestations of IE are not always thoroughly looked for, this may be an underestimate. Petechiae may occur, frequently around the clavicle and lower back, and may progress to a generalised purpura, with associated low or normal platelet counts and focal necrosis seen on histology. Splinter haemorrhages are non-specific striations seen in the nails that may be due to IE or, more frequently, traumatic lesions.
Rheumatic heart disease (RHD)
Rheumatic fever is a multi-systemic disease, commonly affecting children, resulting from a Group A Strep throat infection. This leads to a subsequent immune response and widespread inflammation. RHD may be present due to the persistent inflammation of the heart. This can cause valvulopathy and subsequent heart failure or arrhythmias.
Although the prevalence of RHD has reduced significantly in developed countries, it remains a significant cause of morbidity and mortality in developing countries.
Dermatological manifestations may include subcutaneous nodules and erythema marginatum. Erythema marginatum is a rare annular lesion thought to be due to antibodies cross-reacting with keratin complexes. Subcutaneous nodules are also rare and are granulomatous lesions that form as a result of the delayed hypersensitivity reaction to the Group A Strep antigens. Subcutaneous nodules may also persist long after treatment for RHD. The nodules are thought to correlate with presence of cardiac lesions, but are not seen to be associated with severity of arthritis. Skin ulcers may also present as a key focus of Group A Strep in the aetiology of RHD in developing countries.
Cardiac myxoma
Cardiac myxomas are the most common primary cardiac tumour. These lesions are pathologically benign, although they have potentially life-threatening sequelae. These can include obstructive disease within the heart or embolisation to distant organs. Various dermatological manifestations have been described. Embolic phenomena may include cyanosis, petechiae, pruritic erythematous papules or plaques and LR. Autoimmune phenomena, potentially due to interleukin (IL)-6 secretion, may manifest as Raynaud’s and vasculitis.
Furthermore, Carney complex is a well-described syndrome, normally inherited in an autosomal dominant fashion, characterised by hyperpigmentation of the skin and mucosae, myxomas and endocrine overactivity. It accounts for 7% of all cases of cardiac myxomas.11
Cardiac sarcoma
Cardiac sarcomas are a rare malignant tumour affecting the heart. Angiosarcomas are the most common form of cardiac sarcomas and are most frequently found in middle-aged men. This may lead to heart failure, chest pain, shortness of breath and weight loss. Due to the nonspecific presentation of disease and rarity, metastasis is often present at diagnosis. Spread to skin is rare, but may be present in between 3.4 and 6.7% of cases.12 Thus, cardiac sarcomas should be suspected as a potential primary where cutaneous metastases are present. These may present as discrete papular or nodular lesions, which can be erythematous.
LEOPARD syndrome

LEOPARD syndrome (figure 5), first described in 1936,13 is a rare neuro-cardio-facial-cutaneous syndrome that manifests with multiple lentigines, electrocardiographic (ECG) abnormalities, ocular hypertelorism, pulmonary stenosis, genital abnormalities, short stature and hearing impairment. The lentigines, present in over 90% of cases, are most prevalent on the upper part of the trunk and neck. Other skin manifestations have also been described, such as café-au-lait spots and hypopigmented macules.
ECG abnormalities are seen in around 75% of patients and include left ventricular or biventricular hypertrophy, QTc prolongation and repolarisation abnormalities. Hypertrophic cardiomyopathy is seen in up to 80% of patients with cardiac defects, and represents a potentially life-threatening complication of the syndrome. Cardiac abnormalities often precede the presence of lentigines. However, the appearance of lentigines may lead to a progression in the hypertrophy.
Kawasaki disease
Kawasaki disease is an acute systemic vasculitis, which was first characterised in 1967.14 It has a typical onset under the age of five years, often with prolonged febrile illness and nonspecific symptoms of acute inflammation. It is the leading cause of acquired cardiac disease in children in the developed world, and cardiac complications can include valvular disease, myocarditis, pericarditis and coronary artery aneurysm (CAA). CAA may be present in 20–25% of those untreated, and can lead to myocardial infarction, rhythm abnormalities or even sudden death. Management with antipyretics, intravenous immunoglobulins (IVIG) and aspirin may be commenced to reduce the risk of complications. Prompt treatment with IVIG may reduce the risk of CAA development to around 5%.15
Kawasaki disease may manifest in other organ systems before serious cardiac complications. These include arthritis, lymphadenopathy, diarrhoea and abdominal pain, extreme lethargy and malaise, hydrops of the gallbladder and urethritis. Dermatological manifestations are varied. These include a rash, which may be morbilliform or macular. The rash often manifests during the early febrile phase and disappears with resolution of the fever. Mucous membranes are affected with oral and pharyngeal erythema, cracked lips and the classical strawberry tongue. Palmar erythema, oedema of the dorsum of the hands and feet and/or periungual desquamation may be seen in the extremities. Beau’s lines, which are deep transverse ridges in the nails, may be seen. If the child has been inoculated with BCG (Bacillus Calmette–Guérin), an erythematous rash or induration may be seen around the site of vaccination. Ocular examination may reveal bilateral bulbar conjunctival injection with uveitis.
Iatrogenic disease
Cardiac transplantation
Heart transplants (HT) are performed for a number of different indications. The risk of mortality from the operation and its complications is significant and, thus, the benefits must be weighed up against the risks. Numerous dermatological complications of HT are noted.
The increased risk of skin cancer is well established after solid organ transplantation. This increased risk may be higher in HT patients than kidney transplant patients.16,17 In Western countries, skin cancer is the most common malignancy post-transplant, with the incidence increasing with time post-transplant. Squamous cell carcinoma (SCC), in particular, is seen to have an increased risk, although the incidence of basal cell carcinoma (BCC), melanoma and pre-malignant lesions is also increased. Rarer adnexal tumours, such as trichilemmal carcinomas, have also been described in HT patients, potentially precipitated by immunosuppression. These tumours are locally invasive and rarely metastasise, but their appearance is similar to SCCs (clear cell type) often leading to misdiagnosis. A prominent connective tissue sheath is seen on histology of trichilemmal carcinomas helping to differentiate the lesions. SCCs may also be more aggressive and have a greater risk of metastasis in transplant recipients.
Several risk factors exist for skin cancer in HT patients. Skin cancers tend to be found on sun-exposed sites and are seen more often on those with Fitzpatrick type I or II (pale/fair skin that burns easily) skin. Interestingly, human leukocyte antigen (HLA) mismatch has been seen to be associated with a reduction in skin cancer risk,18 most likely due to the activation of the immune system in tumour surveillance. Furthermore, the combination of immunosuppressants and dosage regimen used may also affect skin cancer risk in transplant recipients.
Infection is also a major complication of HT and may lead to significant mortality, morbidity and increased length of hospital admission. Skin and soft tissue infections are common and prompt treatment may be life-saving. Immunosuppression leads to increased risk of several pathogens, in addition to those commonly encountered in post-surgical and cardiac patients. Bacterial infection can present as cellulitis or folliculitis. Various fungal infections may be present including Candida albicans, pityriasis versicolor from Malassezia and rarer infections, such as Alternariosis.
Malakoplakia, a rare and chronic inflammatory disorder, which may affect the skin as painful papules, plaques or nodules, has also been described in HT patients, likely secondary to immunosuppression. Other dermatological features may also be present post-transplant. These include hypertrichosis, seborrhoeic dermatitis, warts and dermatological side effects of steroids.
Cardiac devices
Cardiac devices may be placed for a variety of reasons and include implantable cardioverter devices, pacemakers and implanted loop recorders. Dermatological complications may include keloid and hypertrophic scars at incision sites and contact dermatitis from the devices. As with HT scars, cutaneous injury may precipitate certain dermatological diseases, such as psoriasis, vitiligo and lichen planus, as described by the Koebner phenomenon.
Multi-systemic conditions
Various multi-systemic disorders may affect multiple organ systems including the skin and cardiovascular system. An in-depth review of these conditions is beyond the scope of this article. However, it is important to note connective tissue disorders such as Marfan’s syndrome, Ehlers-Danlos and pseudoxanthoma elasticum have serious cardiac sequelae, and dermatological examination may provide key clues to the underlying disease process. Moreover, systemic amyloidosis, sarcoidosis and rheumatological diseases, such as systemic sclerosis, dermatomyositis and SLE, all have multiple cardiac complications and dermatological manifestations. These disease processes may present to clinicians in multiple specialities and often require multi-disciplinary management.
Cardiac manifestations of dermatological disease
Here, we have focused on the dermatological manifestations of cardiac disease. However, it is important to appreciate that dermatological disease may also have an impact on cardiovascular health. For instance, psoriasis has been seen to be associated with a significantly increased risk of developing IHD, cerebrovascular events and peripheral vascular disease.19 Furthermore, it is associated with the metabolic syndrome, with severe psoriasis conferring a greater risk than mild psoriasis.20 Thus, the link between the dermatological and cardiovascular system is complex, and an improved understanding of the relationship may further our understanding of the underlying disease processes.
Conclusion
In summary, several cardiac conditions have dermatological manifestations that may provide clues as to the underlying disorder and its severity. This review highlights the benefits of being vigilant to such signs and conducting a thorough dermatological examination. We note that, although several case reports exist in the literature, few larger studies have been published. Moreover, we envisage greater interdisciplinary liaison would be beneficial to further elucidate the link between the skin and the cardiovascular system, and ultimately improve our understanding of the underlying disease processes and the patient care we can offer.
Key messages
- Several cardiac conditions have dermatological manifestations that may aid diagnosis and guide investigations and management
- The dermatological history and examination can be a key aspect of the assessment of a patient presenting with cardiovascular symptoms
- Greater interdisciplinary liaison will improve our understanding of the association between the two important systems
Conflicts of interests
None declared.
Funding
None.
Patient consent
Written patient consent was obtained for figures 1 and 3.
Note
Further references are available from the authors on request.
References
1. Nair PA, Singhal R. Xanthelasma palpebrarum – a brief review. Clin Cosmet Investig Dermatol 2018;11:1–5. https://doi.org/10.2147/CCID.S130116
2. Yamada T, Hara K, Umematsu H, Kadowaki T. Male pattern baldness and its association with coronary heart disease: a meta-analysis. BMJ Open 2013;3:e002537. https://doi.org/10.1136/bmjopen-2012-002537
3. Sari I, Aykent K, Davutoglu V et al. Association of male pattern baldness with angiographic coronary artery disease severity and collateral development. Neth Heart J 2015;23:265–74. https://doi.org/10.1007/s12471-015-0688-3
4. ElFaramawy AAA, Hanna IS, Darweesh RM, Ismail AS, Kandil HI. The degree of hair graying as an independent risk marker for coronary artery disease, a CT coronary angiography study. Egypt Heart J 2018;70:15–19. https://doi.org/10.1016/j.ehj.2017.07.001
5. Frank ST. Aural sign of coronary-artery disease. N Engl J Med 1973;289:327–8. https://doi.org/10.1056/NEJM197308092890622
6. Pasternac A, Sami M. Predictive value of the ear-crease sign in coronary artery disease. Can Med Assoc J 1982;126:645–9. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1863259/
7. Lucenteforte E, Romoli M, Zagli G, Gensini GF, Mugelli A, Vannacci A. Ear lobe crease as a marker of coronary artery disease: a meta-analysis. Int J Cardiol 2014;175:171–5. https://doi.org/10.1016/j.ijcard.2014.04.025
8. Davis TM, Balme M, Jackson D, Stuccio G, Bruce DG. The diagonal ear lobe crease (Frank’s sign) is not associated with coronary artery disease or retinopathy in type 2 diabetes: the Fremantle Diabetes Study. Aust N Z J Med 2000;30:573–7. https://doi.org/10.1111/j.1445-5994.2000.tb00858.x
9. Agouridis AP, Elisaf MS, Nair DR, Mikhailidis DP. Ear lobe crease: a marker of coronary artery disease? Arch Med Sci 2015;11:1145–55. https://doi.org/10.5114/aoms.2015.56340
10. Matsui Y, Okada N, Nishizawa M et al. An Osler’s node and a Janeway lesion. Intern Med 2009;48:1487–8. https://doi.org/10.2169/internalmedicine.48.2377
11. Reynen K. Cardiac myxomas. N Engl J Med 1995;333:1610–17. https://doi.org/10.1056/NEJM199512143332407
12. Val-Bernal JF, Figols J, Arce FP, Sanz-Ortiz J. Cardiac epithelioid angiosarcoma presenting as cutaneous metastases. J Cutan Pathol 2001;28:265–70. https://doi.org/10.1034/j.1600-0560.2001.028005265.x
13. Zeisler EP, Becker SW. Generalized lentigo: its relation to systemic nonelevated nevi. Arch Derm Syphilol 1936;33:109–25. https://doi.org/10.1001/archderm.1936.01470070112010
14. Kawasaki T. [Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children]. Arerugi 1967;16:178–222.
15. Friedman KG, Gauvreau K, Hamaoka-Okamoto A et al. Coronary artery aneurysms in Kawasaki disease: risk factors for progressive disease and adverse cardiac events in the US population. J Am Heart Assoc 2016;5:e003289. https://doi.org/10.1161/JAHA.116.003289
16. Euvrard S, Kanitakis J, Pouteil-Noble C et al. Comparative epidemiologic study of premalignant and malignant epithelial cutaneous lesions developing after kidney and heart transplantation. J Am Acad Dermatol 1995;33(2 Pt 1):222–9. https://doi.org/10.1016/0190-9622(95)90239-2
17. Fortina AB, Caforio AL, Piaserico S et al. Skin cancer in heart transplant recipients: frequency and risk factor analysis. J Heart Lung Transplant 2000;19:249–55. https://doi.org/10.1016/S1053-2498(99)00137-0
18. Gao Y, Twigg AR, Hirose R et al. Association of HLA antigen mismatch with risk of developing skin cancer after solid-organ transplant. JAMA Dermatol 2019;155:307–14. https://doi.org/10.1001/jamadermatol.2018.4983
19. Prodanovich S, Kirsner RS, Kravetz JD, Ma F, Martinez L, Federman DG. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol 2009;145:700–03. https://doi.org/10.1001/archdermatol.2009.94
20. Langan SM, Seminara NM, Shin DB et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol 2012;132(3 Pt 1):556–62. https://doi.org/10.1038/jid.2011.365
