Imagine that it is possible to know, the actual coronary blood flow. Would this not remove any doubt, if a chest pain is the heart’s fault?
Introduction

The heart receives 5% or 250 ml/min of cardiac output and extracts 75% of oxygen delivered, even under basal conditions, compared with less than 5% for the skeletal muscle, therefore, when oxygen requirement rises, the only way to match this is for coronary flow to increase.1 This review concerns new techniques for estimating coronary flow, against a brief historical backdrop and a concise overview of coronary haemodynamics relevant to coronary microvascular dysfunction (CMD), an exhaustive rendition of which is published elsewhere.2
Angina and coronary artery disease
Angina pectoris, which in Latin literally means ‘strangling sensations in the chest’, was coined just over 250 years ago by William Heberden in 1768, an English physician at St. George’s Hospital in London, although he is better known for Heberden’s nodes, the osteoarthritic distal finger joints, which are a clinical sign well-rehearsed among medical students the world over. His detailed description of angina has not been bested since, and is well worth revisiting.3
Another well-known St. George’s doctor, John Hunter, a Scot who ushered in modern surgery with his deep knowledge of human anatomy, was a sufferer of angina, and died of myocardial infarction (MI) during heated exchanges in the boardroom, when he tried to secure a job for his protégé at the hospital in 1793.4 Edward Jenner, who seemingly a Gloucestershire countryside family doctor, of smallpox vaccination fame, was Hunter’s apprentice at St. George’s, postulated that coronary artery disease presents in a spectrum from angina to cardiac death. He, however, kept his opinion to himself, initially, so as to avoid upsetting his mentor, because the only treatments at the time were opium and bedrest.3 These doctors’ names are today immortalised by the Heberden Ward, Hunter and Jenner Wings at St. George’s. Now we know angina is an interplay between ‘supply and demand’ for delivery of oxygen to match the metabolic needs of the heart. All things being equal and simply stated, it is all about blood ‘flow’ to the heart through a network of coronary arteries into diminishingly small vessels, practically divided into the epicardial coronary artery and the microvasculature.
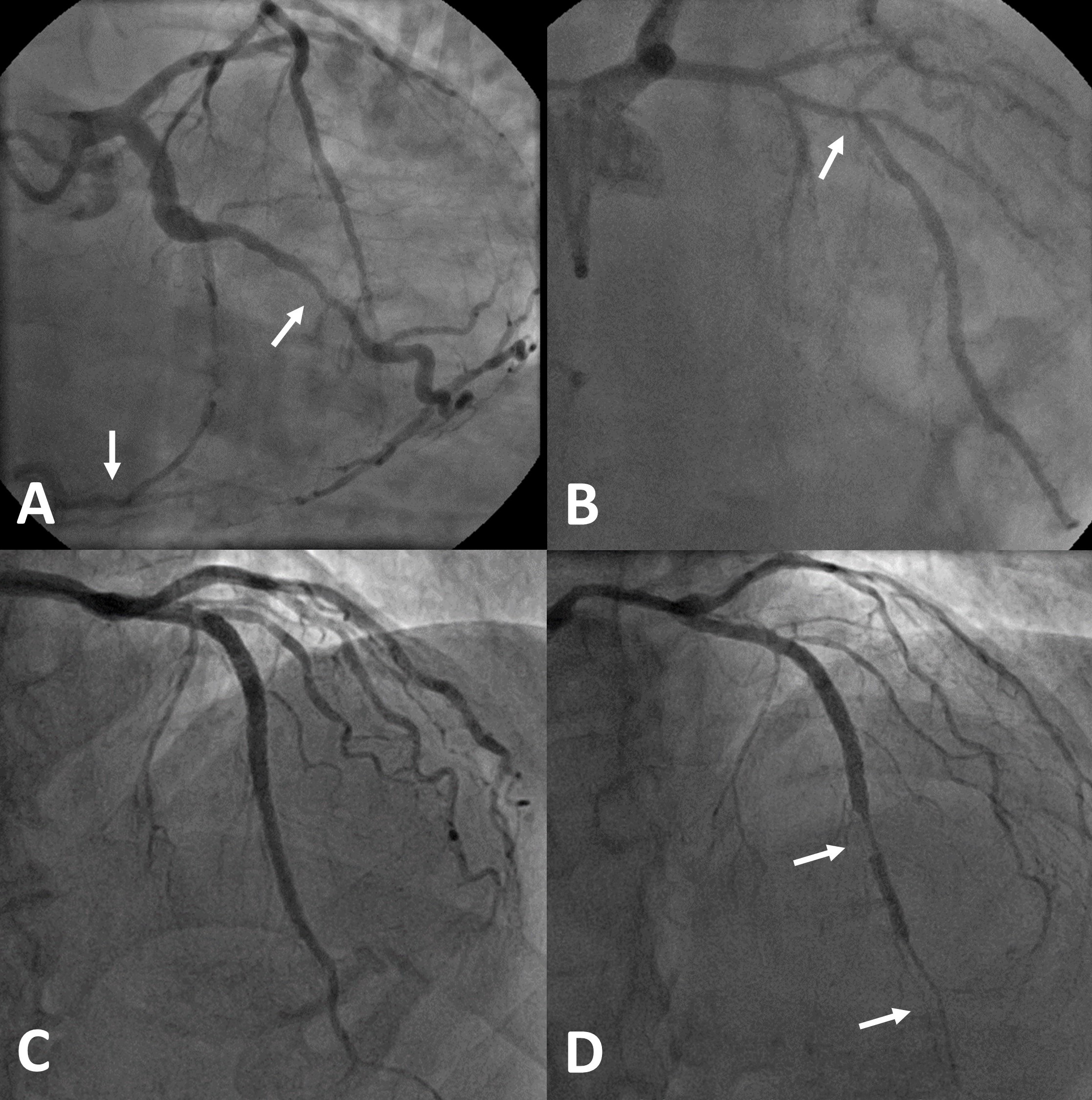
The epicardial coronary artery functions as conduit and puts up little resistance to flow. On the other hand, the microvasculature draws blood in by modulating resistance, as well as aided by left ventricular (LV) diastole, allowing autoregulation to maintain blood flow into the heart across a mean blood pressure range of 60–150 mmHg in accordance with Ohm’s law (flow = pressure/resistance).1 In other words, the higher the perfusion pressure, the greater the flow, but it inversely mirrors resistance generated within the microvasculature, not unlike the ‘river–dam–reservoir’ analogy, i.e. the water level in the reservoir is decided by how much water is let in by raising or lowering the dam, regardless of the state of the feeding river. When there is epicardial coronary stenosis, the microvascular resistance falls to maintain flow despite low perfusion pressure, hence, the disconnect between epicardial coronary artery disease, symptoms and even outcome (figures 1A and 1B).5,6 The corollary of this is that up to a third of patients post-coronary stenting continue to suffer angina, as flow could still be limited by unmasking pre-existing high microvascular resistance.7 Indeed, co-existent CMD in these patients carries adverse prognosis.8
Cardiac syndrome X
The label ‘syndrome X’ was first used by Harvey Kemp in 1973 to describe atrial-pacing induced cardiac ischaemia in a group of patients with angiographically unobstructed coronary arteries.9 Atrial-pacing has also been used to uncover rate-dependent left bundle branch block as a cause of exercise-induced angina.10 There is recent proliferation in acronyms to replace the syndrome X designation, such as ANOCA, INOCA and MINOCA (A – angina, I – ischaemia, MI – myocardial infarction, and NOCA – non-obstructive coronary artery), without addressing the underlying aetiologies, and there is a sizable group of such patients who comprise 25–50% of those undergoing coronary angiogram for angina.11,12 Myron Printzmetal first described angina that occurs at rest in 1959, and the vasospastic aetiology was elucidated by Attilio Maseri’s team in Rome in 1975.13 Maseri, who recently passed aged 85 in Milan on 3 September 2021, in addition, provided proof for the efficacy of nitrates in relieving angina and worked out reliable coronary flow estimation in the form of [15O]H2O positron emission tomography (PET), which is the current non-invasive gold standard.14 One of his trainees in the 1980s at Hammersmith Hospital in London, Juan-Carlos Kaski, moved to St. George’s in 1991 and led the academic cardiology department into major collaborative research in CMD.12
Adenosine, cardiac ischaemia and coronary blood flow
Angina is a referred visceral pain transmitted via the autonomic nervous system. Therefore, patients with denervated transplanted heart, those who had thoracic sympathectomy and some diabetics, do not experience it, and this also suggests that nervous control of coronary flow is relatively modest.1 In general, when coronary flow is compromised, cardiac ischaemia leads to build up of tissue adenosine, an adenosine triphosphate by-product, which is the mitochondrial fuel from fatty acid phosphorylation or glucose glycolysis, the latter predominates during ischaemia occurring anaerobically. Hence, lactate, a derivative of the process, is sometimes measured in the coronary sinus as a marker for cardiac ischaemia, although this is not reliable for CMD. Adenosine causes potent vasodilation by activating the adenosine receptors, and the subtype A1-receptor sends signals along the cardiac vagus afferent nerves, which travel up the spinothalamic tracts corresponding to T1–5 in the spinal cord, as well as subnucleus caudalis 5 in the brain stem via the cervical vagus nerves. These tracts, incidentally, also receive somatic dermatomal sensations from chest, upper arm and jaw, convergently perceived as angina in the brain by lighting up the thalami, as well as prefrontal and, presumably, cortices corresponding to homunculus of referred dermatomes.
This adenosine-angina signal is amplified by substance P, which is released by cell necrosis during MI, thus, the increased pain intensity. Incidentally, incremental doses of intracoronary adenosine, up to 20 mg, which has a half-life of under 10 seconds, had been given rapidly to reproduce angina that lasts for one minute under controlled research conditions. Therefore, it would be intriguing to test the typicality of symptoms in some patients with high burden of morbidity, but ethical approval is needed. Given at 50 µg boluses it is a treatment for coronary ‘no reflow’ following balloon dilation or stenting to relieve microvascular spasm from distal embolisation of atherosclerotic crud. It is also routinely used to induce instantaneous hyperaemia for pressure-wire study to assess epicardial coronary stenosis, albeit 100–200 µg would suffice for this purpose.
Aminophylline, a soluble theophylline-ethylenediamine salt in two-to-one ratio, which non-selectively binds to adenosine receptors as an antagonist, relieves angina at the source in CMD but worsens ischaemia through vasoconstriction at higher dosages. It was first-line treatment for acute heart failure given by slow intravenous infusion, through its chronotropic, inotropic, bronchodilator and loop-diuretic potentiating effects. However, because of its narrow therapeutic index for toxicity, frequent drug interactions, and the plethora of modern cardiac drugs, its use in cardiology is curtailed. Nevertheless, it reverses ‘adenosine-induced and atropine-resistant’ heart block in inferior MI due to occlusion of the right coronary artery that supplies the atrioventricular node, when intracoronary low-dose 20 mg boluses are given. Along the same vein, complex coronary angioplasty with rotablation or thrombectomy to the same artery could also cause heart block from calcific debris or clot embolisation, and this is preventable with upfront intravenous 250 mg aminophylline pre-treatment over 10 minutes.
There are degrees of ischaemia, mild ischaemia manifests as angina and more severe ischaemia causes LV dysfunction, therefore, a negative non-invasive test result, such as stress echocardiogram, does not rule out cardiac ischaemia. Indeed only 50% of patients with CMD exhibit ischaemia on non-invasive tests. Definitive CMD work-up requires invasive coronary physiological assessment. However, measuring coronary flow in the catheter laboratory was a challenge, but Nico Pijls’s team in Eindhoven nevertheless oversaw innovations in this area from pressure-wiring, bolus- to continuous-thermodilution techniques. It is well recognised that how quickly contrast injected down the coronary artery clears relates to the state of the microvasculature. Taking this observation further, in 2001, Pijls’ team15 investigated the average time taken for a 3 ml bolus of saline to traverse the coronary artery, detected using a combined pressure-temperature guidewire-sensor as a measure of coronary flow, and compared this with flow-velocity from doppler-wiring, the standard since 1992, but a finicky and laborious technique. Pijls continued his search for a better method, culminating with the saline continuous-thermodilution technique in 2016.
Endothelium as a continuum and coronary microvascular dysfunction
CMD occurs in two overlapping but distinct ways, functional or structural, and therapies should be targeted accordingly. The endothelium within both the epicardial coronary artery and the microvasculature releases a whole host of vasoactive agents, beyond the scope of this review, that keep these vessels in a semi-vasodilated state. Importantly, the flow down the coronary artery is pulsatile with changes in diameter by as much as 5% from intravascular ultrasound studies, this vessel wall shear stress that is transmitted throughout the vasculature activates the endothelium. Coronary stent, which splints the artery and reduces this pulsatility, therefore, exacerbates endothelial dysfunction, potentially precipitating coronary artery spasm (figures 1C and 1D), as well as a similar effect through upregulation of Rho-kinase in adjacent unstented segments, which phosphorylates the myosin-light-chain rendering the vascular smooth muscle cells sensitive to intracellular calcium ions. This could be specifically inhibited by fasudil, which is classed as a calcium-channel blocker.
Hence, despite the recommended systematic stenting in MI, a stent-sparing strategy is pursued by some researchers, leading to a stenting rate of 60% in the Erosion (Effective Anti-Thrombotic Therapy Without Stenting: Intravascular Optical Coherence Tomography-Based Management in Plaque Erosion) III study.16 Use of high-definition intra-coronary imaging with optical coherence tomography further reduces this to 40%. This incidentally helps to risk stratify MINOCA patients, as many turn out to have plaque erosion without rupture, with overlying thrombus amenable to medical therapy.
There appears to be a gender disparity in CMD and the WISE (Women’s Ischemia Syndrome Evaluation) registry, over the last 25 years, has elucidated this phenomenon and found that the prognosis is not benign, with doubling of mortality to nearly 20% over 10 years.17 In women, oestrogen maintains vascular health and is a powerful endothelium-dependent vasodilator, hence, in the first-half of menstrual cycle when oestrogen is at an ebb, there could be increased vasoreactivity predisposing to angina.12 Further, following menopause, oestrogen deficiency potentiates central release of catecholamines, which surge in the early hours of morning precipitating angina, and also might explain the preponderance of Takotsubo or stress cardiomyopathy from intense microvascular spasm in women due to strong situational emotional triggers.
There are a set number of ways the microvasculature could histopathologically remodel; intimal thickening and medial smooth muscle cell proliferation causing luminal loss, interstitial and perivascular fibrosis restricting vessel dilatation, and capillary rarefaction due either to embolic plugs or increase in muscle mass impeding perfusion. Oxidative stress from reactive oxygen species drive these processes and they are prevalent with the traditional risk factors for coronary artery disease, such as hypertension, diabetes, hyperlipidaemia, smoking, and ageing. Also, any cardiac muscle disease, such as hypertrophic cardiomyopathy, Fabry’s disease and infiltrative amyloidosis, could cause CMD through adverse remodelling of the microvasculature.
Left ventricular diastolic suction
The heart is a ‘Francisco Torrent-Guasp’ muscular band, as homage to its discoverer in 1972, folded origamically into a figure of eight spiral, starting from pulmonary trunk, then right ventricular band, followed by LV apical wrap, next LV basal wrap and finally LV outflow tract. Contraction occurs along the same course, causing clockwise twisting in systole and counter-clockwise untwisting in diastole of LV, with LV apex in fixed position, hence, simulating an inverted piston-in-motion, not unlike that of a car engine. Therefore, in diastole, via the piston phenomenon, the LV sucks blood in to fill. When the shape of the LV apex distorts from any disease process, LV filling becomes pressure-driven and more actively filled via left atrial contraction causing raised LV filling pressure, left atrial dilatation and, eventually, atrial fibrillation precipitating cardiac decompensation. In turn, the raised LV filling pressure raises microvascular resistance. This effect is elegantly demonstrated in patients with aortic stenosis following transaortic valve implantation, microvascular resistance falls accordingly.18
In essence, high LV filling pressure causes CMD indirectly because transmyocardial perfusion pressure is simply the difference between diastolic aortic pressure and LV filling pressure. Similarly, in acute MI despite coronary revascularisation, infarct size continues to extend, thought to be primarily due to reperfusion injury. However, recent works on LV unloading with LV assist devices suggest elevated post-MI LV filling pressure might contribute to ischaemic CMD, ‘no reflow’ and infarct extension.
An illustrative case – procedural
A 61-year-old woman, who was a smoker with diabetes, hypertension and hyperlipidaemia, was admitted for detailed coronary physiological study. She complained of breathlessness and angina. She had right adrenalectomy for an adrenocorticotropic-dependent tumour, which was enabled by coronary angioplasty to her right coronary artery in 2018. A repeat coronary angiogram one year prior showed no significant epicardial coronary artery disease. Her long-acting oral nitrates were withheld for 48 hours pre-procedure.
Coronary angiography via her left distal radial artery using a 6Fr system shows that her left anterior descending (LAD) artery is unobstructed but appears tortuous with decreasing calibre distally (figure 2A). Initial pressure-wire study (Pressure Wire X, Abbott Vascular, USA) on the LAD was performed with CoroFlow™ software (Coroventis, Uppsala, Sweden). The 3 ml saline bolus-thermodilution technique was first performed at baseline followed by hyperaemia with intravenous adenosine infusion, and the following results were obtained: fractional flow reserve (FFR, myocardial ischaemia <0.80) 0.89, coronary flow reserve (CFR, normal >2.5) 1.8 and index of microvascular resistance (IMR, normal <25) 12. Next, a RayFlow© infusion catheter (Hexacath Inc., Paris, France) was used and saline was infused for continuous-thermodilution at the rate of 20 ml/min to induce hyperaemia, followed by 10 ml/min to calculate rest indices. The corresponding flow, Q (ml/min), was 161 and resistance, R (Wood units), 509, versus 61 and 1,481, respectively, therefore, a CFR of 2.6, and microvascular resistance reserve (MRR) of 2.9, calculated from the flow indices. Next, acetylcholine 2 µg, 20 µg, 100 µg, nitroglycerine 200 µg and verapamil 200 µg were given over one minute each through the catheter, and variation in coronary flow measured with the following results: Q 100, 75, 116, 69, 113; R 927, 1,135, 675, 1,172, 703 and resting distal coronary pressure to aortic pressure ratio (PdPa) 0.92, 0.84, 0.73, 091, 0.86 (see below on interpretation). Finally, her LV end-diastolic pressure was directly measured at rest, which was 8 mmHg, rising to 20 mmHg with sustained handgrip, suggesting diastolic dysfunction. Her central aortic pressure was 140/67 mmHg.
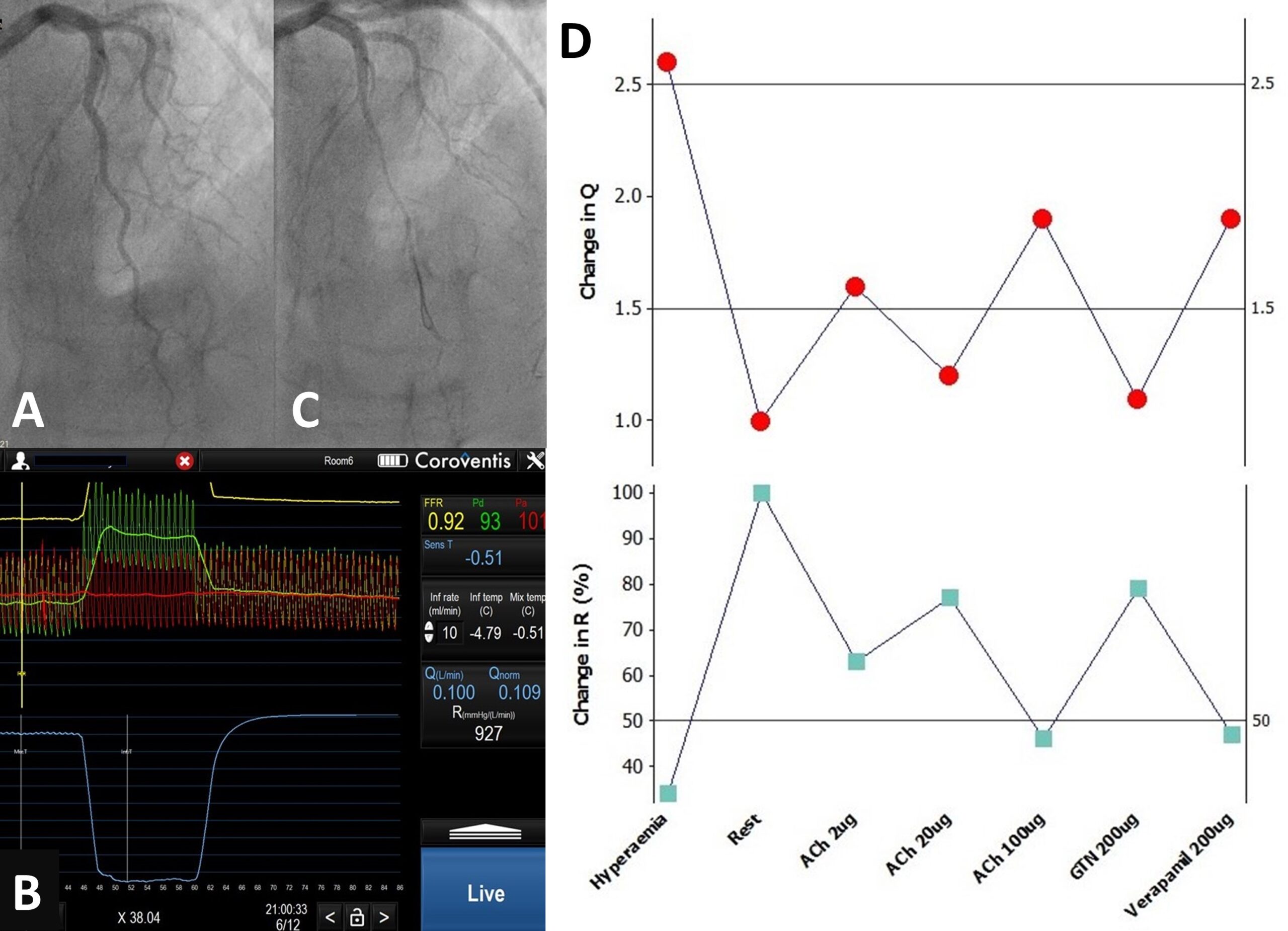
D. Coronary flow and resistance inversely mirroring each other in response to drugs
Bolus-thermodilution versus continuous-thermodilution
CFR/IMR obtained using bolus-thermodilution and adenosine-hyperaemia are currently used to define CMD, with important prognostic implications between patients,11,19 but within the individual, these indices are hard to replicate, limiting their usefulness.20 The two main issues are the subjectivity in picking from multiple transit times of saline boluses at rest and during hyperaemia, and the assumption of hyperaemia with systemic adenosine infusion. Lack of hyperaemia underestimates the CFR and overestimates the IMR. A more vigorous definition of hyperaemia, as applied to perfusion cardiac magnetic resonance, would require adenosine infusion of at least three minutes, with evidence of splenic switch-off, blood pressure fall and increased heart rate response of 10–15 beats/min, although even these requirements are inconsistent, and it is argued that failure to achieve hyperaemia in itself is a marker of CMD.21 Lack of hyperaemia with systemic adenosine could then have three reasons: inadequate adenosine dosing, drug/food interactions or genuine CMD. Therefore, while normal CFR/IMR values are reassuring, abnormal results require confirmation. In contrast, the continuous-thermodilution technique is validated against [15O]H2O perfusion PET for hyperaemic state,22 as well as resting state against doppler-wire technique,23 in measuring coronary flow. Importantly, it is objectively operator-independent and is highly reproducible.24
The continuous-thermodilution technique involves only two additional steps to routine pressure-wire study: (a) saline infusion through the infusion catheter, which allows uniform mixing of saline and blood, and (b) pulling the pressure-wire sensor to the tip of the infusion catheter from mid-vessel, which sits at least 6 cm distally, initially. This allows mixed blood temperature (T) in mid-vessel and saline infusate temperature (Ti) at the catheter tip to be obtained, and the flow, Q, is calculated from 1.08 Qi(Ti/T), where Qi is the rate of saline infusion. The resistance, R, is then derived from Pd/Q, where Pd is the pressure at mid-vessel. These simple steps allow changes in coronary flow and resistance to be obtained in real-time in response to drugs, such as acetylcholine.
An illustrative case – interpretation
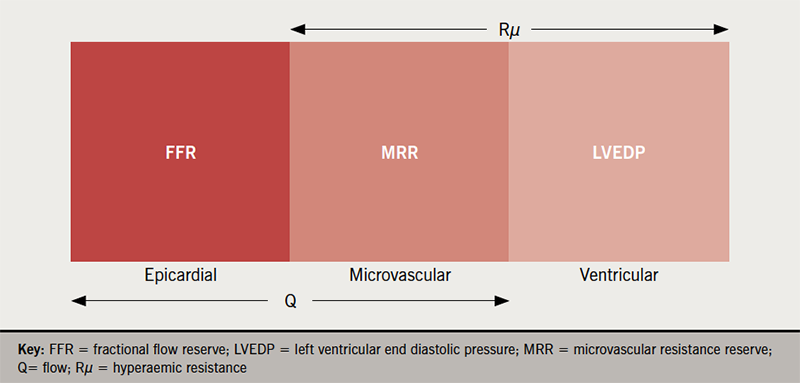
There are four salient observations. First, bolus-thermodilution CFR/IMR, if abnormal, could be verified with absolute coronary flow study with continuous-thermodilution (CFR 1.8 versus 2.6). Second, low-dose acetylcholine could demonstrate normal endothelium-dependent microvascular function as flow increases by 50% (figure 2B). Third, although high-dose acetylcholine provokes significant epicardial coronary artery spasm through endothelial dysfunction (figure 2C), as shown both by angiography and increased pressure-gradient (low PdPa of 0.73), flow ‘paradoxically’ increases where there is intact microvascular functional reserve preventing myocardial ischaemia. This also means that vascular endothelial dysfunction could be patchy rather than generalised, even within the same feeding vessel. Finally, nitroglycerine returns flow back to baseline while verapamil, through endothelium-independent microvascular vasodilation, increases flow, suggesting that it could be a tailored therapy (figure 2D). Taking everything together, this woman has raised ‘minimal’ hyperaemic resistance, Rµ of 509 (<500),25‑27 but preserved MRR of 2.9, in contrast to a low IMR of 12, which could be spurious. Her raised Rµ is likely to be structural in nature, related to her hypertensive heart disease, and it is noteworthy that hand-gripping, a form of resistance exercise, potentially breaches her autoregulatory range with transmyocardial perfusion pressure of <60 mmHg. Figure 3 puts in perspective the various flow indices discussed above. As a result of this study, she was reassured, and her hypertension management could be tightened. She was discharged home with a loop diuretic, which potentially would better control her blood pressure, as well as reducing her symptoms. She was reviewed by teleconsultation two weeks later and her symptoms appear improved.
Summary
Coronary flow autoregulation via the microvasculature is a versatile system and has large reserve to maintain blood flow to the heart despite epicardial coronary artery disease. There is interaction of many factors that causes CMD, a complex and evolving topic, and this could only be elucidated with detailed coronary physiological assessment where reliable blood flow is measured. Highly symptomatic patients with classical angina who are admitted for a diagnostic coronary angiogram should, in the future, be offered one-stop assessment for both epicardial coronary artery disease, as well as in-depth microvascular functional assessment, if the former is unobstructed. This avoids the ANOCA label and potentially endless trials of drug therapy, as many such patients might turn out to have a completely blameless heart, hence, a definitive normal test would focus the mind elsewhere.
Key messages
- Coronary autoregulation could be impaired by coronary microvascular dysfunction, giving rise to angina irrespective of epicardial coronary artery disease, although its absence creates a false dichotomy since coronary microvascular dysfunction is more prevalent in those with epicardial coronary artery disease and left ventricular dysfunction
- Absolute coronary blood flow could be simply measured with the continuous-thermodilution technique
- This novel method allows changes in coronary blood flow in response to acetylcholine and other drugs to be measured in real-time to allow detailed diagnosis of coronary microvascular dysfunction for tailored and targeted therapy
Conflicts of interest
None declared.
Funding
None.
Patient consent
The patients have given informed consent for their cases to be shared for learned communication.
Acknowledgements
The author thanks the Department of Cardiology as led by Drs Rajan Sharma and Manav Sohal, Cardiac Catheter Laboratory Leaders: Mary Keal (Matron), Alexander Grimster (Head of Cardiac Physiology), and Dinesh Sajnani (Head of Cardiac Radiography), Nico Pijls and his team who graciously demonstrated the prototypic continuous-thermodilution technique when the author attended their 19th Aalst-Einthoven Course on 19–20 November 2015, Klio Konstantinou who learnt the technique from Nico Pijls and brought the know-how to St. George’s Hospital in 2021 as a senior trainee, the patients described in this review, and the Departmental and Cardiac Catheter Laboratory auxillary and nursing staff involved in the cases. Lastly, this review was written based on the author’s reading, understanding and interpretation of the complex medical literature, and space allowing only a small number of books and papers are referenced, hence, it was not possible to fully acknowledge all the seminal works done by others, as summarised in this article.
References
1. McLaughlin DP, Wu SS, Stouffer GA. Coronary hemodynamics. In: Stouffer GA, ed. Cardiovascular Hemodynamics for the Clinician. Oxford: Blackwell Publishing, 2008; p.p. 233–24. https://doi.org/10.1002/9780470692608.ch21
2. Del Buono MG, Montone RA, Camilli M et al. Coronary microvascular dysfunction across the spectrum of cardiovascular diseases: JACC State-of-the-Art Review. J Am Coll Cardiol 2021;78:1352–71. https://doi.org/10.1016/j.jacc.2021.07.042
3. Miller AJ, Upton MT. Comments on history of chest pain. In: Diagnosis of Chest Pain. New York: Raven Press, 1988; pp. 11–18.
4. Moore W. The Anatomist’s Heart. The Knife Man. London: Bantam Press, 2005; pp. 500–35.
5. Al-Lamee R, Thompson D, Dehbi HM et al. Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomised controlled trial. Lancet 2018;391:31–40. https://doi.org/10.1016/S0140-6736(17)32714-9
6. Maron DJ, Hochman JS, Reynolds HR et al. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med 2020;382:1395–407. https://doi.org/10.1056/NEJMoa1915922
7. Niccoli G, Montone RA, Lanza GA, Crea F. Angina after percutaneous coronary intervention: the need for precision medicine. Int J Cardiol 2017;248:14–19. https://doi.org/10.1016/j.ijcard.2017.07.105
8. Nishi T, Murai T, Ciccarelli G et al. Prognostic value of coronary microvascular function measured immediately after percutaneous coronary intervention in stable coronary artery disease: an international multicenter study. Circ Cardiovasc Interv 2019;12:e007889. https://doi.org/10.1161/CIRCINTERVENTIONS.119.007889
9. Kemp HG Jr. Left ventricular function in patients with the anginal syndrome and normal coronary arteriograms. Am J Cardiol 1973;32:375–6. https://doi.org/10.1016/S0002-9149(73)80150-X
10. Czuriga D, Lim PO. Cardiac resynchronization therapy relieves intractable angina due to exercise-induced left bundle branch block without left ventricular systolic dysfunction: a detailed case study. J Cardiovasc Electrophysiol 2016;27:609–12. https://doi.org/10.1111/jce.12911
11. Ford TJ, Ong P, Sechtem U et al. Assessment of vascular dysfunction in patients without obstructive coronary artery disease: why, how, and when. JACC Cardiovasc Interv 2020;13:1847–64. https://doi.org/10.1016/j.jcin.2020.05.052
12. Kaski JC, Eslick GD, Merz CNB. Chest Pain with Normal Coronary Arteries. A Multidisciplinary Approach. London: Springer-Verlag, 2013; pp. 356. https://doi.org/10.1007/978-1-4471-4838-8
13. Maseri A, L’Abbate A, Chierchia S, Parodi O. Coronary artery spasm – diagnostic and therapeutic implications. Am Heart J 1978;96:554–5. https://doi.org/10.1016/0002-8703(78)90171-0
14. Araujo LI, Lammertsma AA, Rhodes CG et al. Noninvasive quantification of regional myocardial blood flow in coronary artery disease with oxygen-15-labeled carbon dioxide inhalation and positron emission tomography. Circulation 1991;83:875–85. https://doi.org/10.1161/01.CIR.83.3.875
15. De Bruyne B, Pijls NH, Smith L, Wievegg M, Heyndrickx GR. Coronary thermodilution to assess flow reserve: experimental validation. Circulation 2001;104:2003–06. https://doi.org/10.1161/hc4201.099223
16. Jia H, Dai J, He L et al. EROSION III: a randomized trial of OCT-guided intervention in STEMI with early infarct artery patency. Presented at Transcatheter Cardiovascular Therapeutics (TCT) conference, November 2021, Orlando, USA. Available from: http://clinicaltrialresults.org/dr-haibo-jia-jingbo-hou-and-dr-c-michael-gibson-discuss-erosion-iii-a-randomized-trial-of-oct-guided-intervention-in-stemi-patients-with-early-infarct-artery-patency
17. Tjoe B, Barsky L, Wei J et al. Coronary microvascular dysfunction: considerations for diagnosis and treatment. Cleve Clin J Med 2021;88:561–71. https://doi.org/10.3949/ccjm.88a.20140
18. Wiegerinck EM, van de Hoef TP, Rolandi MC et al. Impact of aortic valve stenosis on coronary hemodynamics and the instantaneous effect of transcatheter aortic valve implantation. Circ Cardiovasc Interv 2015;8:e002443. https://doi.org/10.1161/CIRCINTERVENTIONS.114.002443
19. Lee JM, Jung JH, Hwang D et al. Coronary flow reserve and microcirculatory resistance in patients with intermediate coronary stenosis. J Am Coll Cardiol 2016;67:1158–69. https://doi.org/10.1016/j.jacc.2015.12.053
20. Pijls NHJ, de Vos AMJ, Keulards DCJ. Measurement of absolute coronary blood flow and microvascular resistance: a new window to coronary microcirculation. J Am Coll Cardiol 2021;77:742–4. https://doi.org/10.1016/j.jacc.2020.12.016
21. Farzaneh-Far A, Wong J. Stressed enough? Hyperaemic thresholds during quantitative cardiovascular magnetic resonance perfusion mapping. Eur Heart J Cardiovasc Imaging 2021;22:282–4. https://doi.org/10.1093/ehjci/jeaa268
22. Everaars H, de Waard GA, Schumacher SP et al. Continuous thermodilution to assess absolute flow and microvascular resistance: validation in humans using [15O]H2O positron emission tomography. Eur Heart J 2019;40:2350–9. https://doi.org/10.1093/eurheartj/ehz245
23. Gallinoro E, Candreva A, Colaiori I et al. Thermodilution-derived volumetric resting coronary blood flow measurement in humans. EuroIntervention 2021;17:e672–e679. https://doi.org/10.4244/EIJ-D-20-01092
24. Xaplanteris P, Fournier S, Keulards DCJ et al. Catheter-based measurements of absolute coronary blood flow and microvascular resistance: feasibility, safety, and reproducibility in humans. Circ Cardiovasc Interv 2018;11:e006194. https://doi.org/10.1161/CIRCINTERVENTIONS.117.006194
25. Fournier S, Keulards DCJ, van ‘t Veer M et al. Normal values of thermodilution-derived absolute coronary blood flow and microvascular resistance in humans. EuroIntervention 2021;17:e309–e316. https://doi.org/10.4244/EIJ-D-20-00684
26. Konst RE, Elias-Smale SE, Pellegrini D et al. Absolute coronary blood flow measured by continuous thermodilution in patients with ischemia and nonobstructive disease. J Am Coll Cardiol 2021;77:728–41. https://doi.org/10.1016/j.jacc.2020.12.019
27. Rivero F, Gutierrez-Barrios A, Gomez-Lara J et al. Coronary microvascular dysfunction assessed by continuous intracoronary thermodilution: a comparative study with index of microvascular resistance. Int J Cardiol 2021;333:1–7. https://doi.org/10.1016/j.ijcard.2021.03.005
