The coronary artery calcium (CAC) score is a marker of advanced coronary atherosclerosis. Numerous prospective cohorts have validated CAC as an independent marker that improves prognostication in atherosclerotic cardiovascular disease (ASCVD) beyond traditional risk factors. Accordingly, CAC is now incorporated into international cardiovascular guidelines as a tool to inform medical decision-making. Particular interest concerns the significance of zero CAC score (CAC=0). While many studies report CAC=0 to virtually exclude obstructive coronary artery disease (CAD), non-negligible rates of obstructive CAD despite CAC=0 are reported in certain populations. Overall, the current literature supports the power of zero CAC as a strong downward risk classifier in older patients, whose CAD burden predominantly involves calcified plaque. However, with their higher burden of non-calcified plaque, CAC=0 does not reliably exclude obstructive CAD in patients under 40 years. Illustrating this point, we present a cautionary case of a 31-year-old patient found to have severe two-vessel CAD despite CAC=0. We highlight the value of coronary computed tomography angiography (CCTA) as the gold-standard non-invasive imaging modality when the diagnosis of obstructive CAD is in question.
Introduction
Since its inception by Agatston and Janowitz in 1990, coronary artery calcium (CAC) scoring has blossomed from a novel imaging tool to an internationally accepted biomarker of cardiovascular risk included in current preventive atherosclerotic cardiovascular disease (ASCVD) guidelines.1-7 With its growing adoption, debate has emerged over the proper use of CAC scores in risk stratification, with controversy surrounding its role in excluding obstructive disease in symptomatic patients. Presently, we focus on the role of CAC in risk stratification for coronary heart disease (CHD). We review the overwhelming evidence validating CAC as a diagnostic and prognostic indicator in older adults but highlight its unreliability in younger patients under 40 years. We conclude with a cautionary case of a 31-year-old man admitted to our hospital who was found to have severe two-vessel coronary artery disease (CAD) despite zero CAC score.
Clinical overview
CAC as a CHD risk marker: the view from clinical end point studies
A nascent field of clinical imaging research investigating CAC grew throughout the 1990s,8 and culminated in the publication of foundational end point studies over the subsequent decade, establishing CAC’s utility as a marker of CHD (table 1).1,2,9-14 This work established, not only a proportional relationship between CAC score and incident CHD risk, but demonstrated CAC’s ability to improve CHD prediction independent of traditional risk factors, such as those included in the Framingham risk score (FRS).15 Greenland et al. found a 3–9% increase in 10-year coronary event risk beyond FRS criteria in individuals with CAC score over 300.14 The Rotterdam study found CAC scores improved prediction of incident CHD, ASCVD, and all-cause mortality beyond traditional risk factors among 1,795 asymptomatic adults over 70 years.10 The multi-ethnic study of atherosclerosis (MESA) extended these findings to a diverse cohort including Black (28%), Hispanic (22%), Chinese (12%), and White (39%) patients.13 MESA found an 18–39% increase in coronary event risk associated with each doubling of the CAC score, without major differences between ethnic groups.13 The Framingham Offspring study further validated these observations among 3,486 descendants of the original Framingham Heart Study.16 Again, CAC improved CHD prediction beyond Framingham risk factors, and correctly reclassified 85% of patients initially deemed at intermediate risk for CHD.16 Notable cohort studies establishing the value of CAC in ASCVD risk assessment are summarised in table 1.
Table 1. Notable studies establishing coronary artery calcium (CAC) as a biomarker in atherosclerotic cardiovascular disease (ASCVD) risk assessment
| Study | Year | Country | Patients | Population | Age, years | Follow-up, years | Key findings | |
|---|---|---|---|---|---|---|---|---|
| Rotterdam10 | 2005 | Netherlands | 1,795 | Asymptomatic adults | 71 (62–85) | 3.3 ± 0.8 | Adding CAC score to FRS improved CHD risk prediction | |
| Prospective Army Coronary Calcium (PACC) Project9 | 2005 | USA | 2,000 | Asymptomatic active-duty military | 42.9 ± 2.8 (40–50) | 3.0 ± 1.6 (1–6) | Hazard ratio increase of 4.3 for incident CAD per CAC tertile. CAC>0 in men associated with relative risk of 12 for incident CAD |
|
| Cooper Clinic Cohort11 | 2005 | USA | 10,746 | Asymptomatic adults | 53.8 ± 9.9 (22–96) | 3.5 ± 1.4 | Dose-dependent relationship between CAC score and incident CHD surviving adjustment for traditional risk factors. CAC associated with CHD in both younger (<40) and older (>65) patients | |
| St. Francis Heart Study12 | 2005 | USA | 4,903 | Asymptomatic adults | 59 ± 6 | 4.3 | CAC predicted incident CAD better than FRS | |
| MESA13 | 2008 | USA | 6,722 | Asymptomatic multi-ethnic adults | 62.2 ± 10.2 (45–84) | 3.8 | Doubling of CAC score associated with 15–35% increased risk of major coronary event and 14% relative increase in incident ASCVD | |
| Heinz Nixdorf Recall Study19 | 2010 | Germany | 4,129 | Asymptomatic adults without known CAD | 59 ± 8 (45–75) | 5.1 ± 0.3 | Reclassifying intermediate risk patients based on FRS to low risk (if CAC<100) or high risk (if CAC≥400) categories improved prediction of incident coronary events | |
| Dallas Heart Study69 | 2015 | USA | 2,084 | Multi-ethnic adults without diabetes or CVD | 44.4 ± 9.0 | 9.2 ± 1.3 | CAC score improved CHD risk classification in younger adults | |
| BioImage63 | 2016 | USA | 5,805 | Adults without known CVD | 68.9 ± 6.0 | 2.7 | CAC-guided reclassification of CHD risk achieved a 22% improvement in specificity with no loss in sensitivity, driven by down-classifying risk among patients with CAC=0 | |
| Jackson Heart Study70 | 2016 | USA | 2,944 | African-American adults | 60 (21–64) | – | Adding CAC score to FRS improved prediction of CVD prevalence | |
| Framingham Offspring16 | 2016 | USA | 3,486 | Men ≥35 y, Women ≥40 y | 50 ± 10 | 8 | CAC improved prediction of incident CHD beyond traditional risk factors and accurately reclassified 2/3 of intermediate-risk patients | |
| CARDIA71 | 2017 | USA | 5,115 | Black and White younger adults | 40.3 ± 3.6 (at study year 15) | 12.5 | CAC>0 associated with 5-fold increase in CHD incidence after adjustment for baseline risk. CVD risk factors in early adult life identified patients who later developed CAC | |
| CAC Consortium72 | 2020 | USA | 66,636 | Adults without known CHD | 54 ± 11 | 12.5 | CAC associated with CHD-attributable, CVD-attributable and all-cause mortality in a dose-dependent manner | |
| Key: ASCVD = atherosclerotic cardiovascular disease; CAC = coronary artery calcium; CAD = coronary artery disease; CHD = coronary heart disease; CVD = cardiovascular disease; FRS = Framingham risk score |
||||||||
CAC and the ‘power of zero’
CAC’s prognostic power to improve cardiovascular risk classification had major implications. Among end point studies, excellent cardiovascular outcomes were noted among patient subgroups with zero detectable CAC (CAC=0). Composite analysis of 16,106 asymptomatic patients with CAC=0 spanning 13 early observational studies found an annual coronary event rate of 0.027%, translating to a negative predictive value (NPV) of 98.1% over a mean follow-up of 4.7 years.17 Other major studies reported annual event rates as low as 0.06–0.16% in asymptomatic adults without detectable CAC.9,12,18-20 Initial results from the CAC Consortium cohort found yearly all-cause mortality of 0.87% among 19,898 asymptomatic middle-age adults with CAC=0,21 and risk of all-cause mortality doubled in patients with even the lowest levels of detectable calcium (i.e. CAC score of 1–10). At 12-year follow-up, incident CHD-attributable mortality among patients with CAC=0 was only 0.17%.22 Subsequent analysis of the MESA cohort found CAC=0 to be the greatest downward indicator of 10-year CHD and overall ASCVD risk among 13 other laboratory, imaging, and clinical risk markers.23
The profound differences observed in cardiovascular events among patients at either CAC score extreme allow for more accurate reclassification of ASCVD risk. This approach has been validated in numerous studies and is of relevance for patients estimated to be at intermediate, borderline, or even low ASCVD risk based on the pooled cohort equations (PCE).5 For instance, among 2,966 MESA patients who were eligible for statin therapy per the contemporary US cholesterol guidelines, 44% had zero detectable CAC.24,25 Among this subset, the observed 10-year ASCVD event rate was only 4.2 per 1,000 person-years, and CAC=0 reclassified 49% of statin-eligible patients to a 10-year ASCVD risk <5%, below the suggested risk threshold for statin therapy.24 Valenti et al. even proposed a 15-year ‘warranty period’ among asymptomatic adults with CAC=0 who were already at low or intermediate risk by PCE, noting a survival rate of 95.1% after mean follow-up of 14.6 years.26 Ultimately, this prognostic power has led to CAC’s inclusion in the current European guidelines as a cardiovascular risk modifier for asymptomatic patients.27 CAC scoring is also incorporated in the current US cholesterol guidelines as a class IIa recommendation to inform decision-making regarding statin therapy in adults over 40 years at borderline risk,4 although debate remains as to CAC’s role in de-escalating pharmacotherapy.28
CT-based screening for obstructive CAD in the symptomatic patient
While much effort has focused on CAC’s use in asymptomatic populations from a preventive health standpoint, its diagnostic applications in patients with chest pain has also garnered considerable interest. Safe and cost-effective risk stratification of chest pain presents a major challenge in both the ambulatory and emergency setting. Chest pain accounts for over six million emergency room visits in the US annually, with three million patients ultimately discharged with non-cardiac diagnoses.29
Excluding obstructive CAD is of cardinal interest in any patient with chest pain, and particularly in those whose presentation raises suspicion for acute coronary syndrome (ACS). From a non-invasive imaging standpoint, coronary computed tomography angiography (CCTA) is unrivaled in the diagnosis of CAD and grading of coronary luminal stenosis. The ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial reported sensitivity of 94–95% and NPV of 99% for excluding moderate (≥50%) or severe (≥70%) coronary stenosis with CCTA compared with angiography.30 Subsequent studies consistently demonstrated >90% sensitivity and superior performance with CCTA compared with myocardial perfusion imaging for detecting obstructive CAD.31,32
Increasingly, CCTA has been adopted by emergency departments (EDs) in the US to facilitate decision-making in patients undergoing ACS rule-out and, potentially, defer formal angiography. In the ROMICAT-II (Rule Out Myocardial Ischemia/Infarction Using Computer Assisted Tomography) trial, 1,000 patients presenting to the ED with acute chest pain and suspicion for ACS despite negative cardiac enzymes and lack of electrocardiogram (ECG) changes were randomised to undergo early CCTA or standard evaluation.33 Patients who underwent early CCTA benefited from shorter length of stay and higher rates of direct ED discharge, and no cases of ACS went undetected (i.e. no re-presentations at 72 hours post-discharge with subsequent positive work-up).
CCTA’s role as a screening tool for chest pain syndrome is also well-validated in the outpatient setting. The PROMISE (Prospective Multicenter Imaging Study for Evaluation of Chest Pain) trial randomised patients with stable chest pain and intermediate pre-test probability to undergo either CCTA or functional testing.34 Incidence of the primary end point (death, myocardial infarction, or hospitalisation for unstable angina) at follow-up was lower in patients who underwent CCTA (0.9%) versus functional testing (2.1%), despite lower prevalence of normal test results in the CCTA arm (33% vs. 78%). CCTA had superior discriminative ability in event prediction (c-statistic 0.72 vs. 0.64) and higher diagnostic yield compared with functional testing.35 Of note, the SCOT-HEART (Scottish COmputed Tomography of the HEART) trial randomised 4,146 outpatients with stable chest pain to either standard care or standard care plus early CCTA.36 Patients undergoing early CCTA had lower incidence of the composite end point of non-fatal myocardial infarction (MI) or CHD-attributable mortality at five-year follow-up. In terms of resource utilisation, there was no difference in five-year rates of angiography or revascularisation procedures, but patients referred to CCTA were more likely to be initiated on preventive or anti-anginal therapies.36
Many large-scale studies have examined the reliability of CAC in patients with chest pain, and have yielded favourable results. Meta-analysis across 32,477 symptomatic patients found that presence of CAC (CAC>0) was strongly associated with cardiovascular event risk, with pooled risk ratios (RR) of 6.1 for incident ASCVD and 7.9 for all-cause mortality.37 Table 2 lists several well-powered cohort studies evaluating the reliability of CAC=0; many data are derived from studies of symptomatic patients undergoing both CAC scoring and CCTA.38 The CONFIRM (COronary CT Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter Registry) study examined 10,037 symptomatic patients without known CAD who underwent both CAC scoring and CCTA, and found that CAC=0 excluded obstructive CAD with NPV of 96.5% for ≥50% stenosis and 98.6% for ≥70% stenosis.39 The PROMISE trial also included 4,209 patients who underwent CCTA and CAC scoring.34 Among 1,457 patients with CAC=0, only 22 patients (1.5%) had ≥50% stenosis and only seven (0.5%) had ≥70% stenosis on CCTA, corresponding to NPV of 98.5% and 99.5%, respectively.34
Table 2. Notable CAC=0 studies*
| Study | Year | Country | Patients | Population | Age, years | Follow-up, years | Confirmatory imaging modality | Findings in CAC=0 patients |
|---|---|---|---|---|---|---|---|---|
| Knez68 | 2004 | Germany | 2,115 | Symptomatic adults | 62 ± 19 | – | ICA | NPV of 100% (≥50% stenosis) |
| Rubinshtein54 | 2007 | Israel | 668 | Symptomatic adults | 54 ± 12 | – | CCTA | NPV of 93% (≥50% stenosis) |
| CONFIRM39 | 2011 | International | 10,037 | Symptomatic adults | 57 ± 12 | 2.1 | CCTA | NPV of 96.5% (≥50% stenosis), 98.6% (≥70% stenosis) |
| Chang58 | 2011 | USA | 1,049 | Acute chest pain, suspected ACS | 48.1 | 30 days | CCTA | NPV of 77.6% (>50% stenosis). 4/17 patients with CAC=0 and obstructive CAD had AMI. Age <50 70% more likely to have obstructive CAD with CAC=0 |
| Kim73 | 2012 | Korea | 2,088 | Symptomatic adults | 58 ± 10 | 2.8 | CCTA | NPV of 95.7% (≥50% stenosis) |
| Hulten74 | 2014 | USA | 1,145 | Symptomatic adults | 55 ± 12 | 2.4 | CCTA | NPV of 99% (≥50% stenosis), 99.6% (≥70% stenosis) |
| Valenti26 | 2015 | USA | 9,715 | Asymptomatic adults | 53.4 ± 10.5 | 14.6 | – | 15-year warranty period against mortality in individuals at low-to-intermediate risk regardless of age or sex |
| PROMISE34 | 2017 | North America | 4,209 | Symptomatic adults at intermediate risk | 60.6 ± 8.2 | 2.2 | CCTA | NPV of 98.5% (≥50% stenosis), 99.5% (≥70% stenosis) |
| Mittal48 | 2017 | UK | 2,730 | Symptomatic adults | 56.9 ± 12.4 | 5.2 | CCTA | NPV of 99.5% (≥70% stenosis) |
| Walter Reed75 | 2018 | USA | 13,644 | Active-duty military, no prior CVD | 50 ± 8 | 9.4 | – | NNT of 3,571 to prevent MACE with 10 years of statin therapy (NNT=12 for CAC>100) |
| Wang43 | 2019 | UK | 1,753 | Symptomatic adults, suspected stable CAD | 56.8 ± 12.0 | 2.2 | CCTA | NPV of 98.1% (≥50% stenosis) |
| Mortensen60 | 2020 | Denmark | 23,759 | Symptomatic adults | 58 | 4.3 | CCTA | CAC=0 prevalence of 93% (age <40) vs. 5% (age >70). Likelihood ratio of ≥50% stenosis given CAC=0 of 0.68 (age <40) vs. 0.18 (age >70) |
| *Expanded from Gagel et al.2 Included studies are limited to cohorts including ≥500 patients. Key: ACS = acute coronary syndrome; AMI = acute myocardial infarction; CAC = coronary artery calcium; CAD = coronary artery disease; CCTA = coronary computed tomography angiography; CVD = cardiovascular disease; ICA = invasive coronary angiography; MACE = major adverse cardiovascular event; NNT = number needed to treat; NPV = negative predictive value |
||||||||
The CRESCENT (Calcium Imaging and Selective CT Angiography in Comparison to Functional Testing for Suspected Coronary Artery Disease) trial evaluated a tiered approach to anatomic testing among 350 patients with stable CAD.40 Patients randomised to the anatomic testing arm first underwent CAC scoring and proceeded to CCTA only if estimated pre-test probability for obstructive CAD was >70%, or if CAC was present on computed tomography (CT). Among 242 patients randomised to anatomic testing, 98 patients had CAC=0 and none sustained major adverse cardiovascular events (MACE) or required further testing after one year of follow-up.40 The tiered anatomic testing approach was associated with reduced need for further diagnostic testing, lower cumulative testing costs, and shorter time to final diagnosis relative to a functional testing-based strategy.38,40 Similar results were obtained in the CRESCENT-II trial, with zero of 45 patients with CAC=0 suffering MACE or subsequently diagnosed with obstructive disease at follow-up.38,41 Low rates of obstructive CAD among patients with CAC=0 were also found in post-hoc analysis of the SCOT-HEART trial,38,42 with only around 1% MACE incidence after five years of follow-up.42 Wang et al. reported outcomes in a prospective series of 1,753 symptomatic patients with stable CAD.43 CAC=0 achieved a NPV of 98.1% for excluding ≥50% stenosis on CCTA. At two-year follow-up, MACE incidence was only 0.6% (five patients) among 751 patients with CAC=0.43 Sixty-three patients (8.4%) with CAC=0 had subclinical non-calcified plaque on CCTA, but there was zero MACE incidence among this subgroup.43
A subset of studies focused specifically on the reliability of CAC for excluding ACS in the emergency setting. Early case series pointed to the promise of CAC=0, reporting sensitivity of 97–100% for excluding ACS in patients with chest pain.44-46 Bittner et al. reviewed 826 consecutive patients presenting to the ED with acute chest pain.38,47 Among 444 patients with CAC=0, rates of obstructive CAD were very low, with NPV of 99.5% for ≥50% stenosis and 99.8% for ≥70% stenosis;47 however, the exclusion of patients with positive initial cardiac enzymes reduced pre-test probability with an overall ACS rate of only 7.9%.47 Mittal et al. evaluated incidence of obstructive CAD in an observational ED cohort including 2,730 patients undergoing CCTA.48 Among the 52.5% of patients with CAC=0, NPV of 98.3% and 99.5% were achieved for excluding ≥50% or ≥70% stenosis, respectively.48 All 24 patients with ≥50% stenosis on CCTA underwent formal angiography, with flow-limiting stenoses ultimately found in only four patients. Patients with CAC=0 had an annual all-cause mortality of only 0.3%, with zero reported coronary events across over five years of mean follow-up.38,48 Furthermore, the presence of non-calcified plaque on subsequent imaging had no association with mortality among patients with CAC=0.48
While the accuracy of CAC score in predicting obstructive CAD is generally excellent, some studies have reported less favourable results. As noted in the CONFIRM study,39 other early reported rates of obstructive CAD were as high as 7–38% in patients with CAC=0, although these data originated from small retrospective case series.49-54 Even the CONFIRM authors concluded that CAC=0 did not reliably exclude CAD in their symptomatic cohort, given that 3.5% of this subgroup still had ≥50% stenosis on CCTA.39 A meta-analysis by Sarwar et al. found CAC=0 excluded obstructive disease (>50% stenosis) with pooled sensitivity of 98% and NPV of 93% across 18 studies of symptomatic patients undergoing CAC scoring and invasive angiography.55 While promising, such error rates preclude deferring CCTA if reasonable suspicion exists for underlying obstructive disease.
Value of CAC=0 across the lifespan
Discrepant results in the literature raise the question of whether zero CAC score is equally meaningful in all patients. The use of CAC=0 to down-classify obstructive CAD risk is debated, especially in younger symptomatic patients with pre-test risk factors for disease. Evidence increasingly points to age as a critical factor in interpreting CAC score. The biological rationale is intuitive: atherosclerosis progresses over decades,56,57 with intimal calcification occurring late in its course.58,59 Older adults with CAD have accumulated calcified plaque over many decades of life, explaining higher average CAC scores and higher prevalence of detectable CAC relative to younger patients.60 As CAC specifically detects calcified plaque, it is unsurprising that CAC=0 excludes clinical disease more accurately in older patients.
Relative to older patients, most coronary plaque in younger patients with CAD is non-calcified.61 This is supported by MESA and related studies that demonstrate the subsequent appearance and progression of CAC over years on serial CT, in a manner correlating with cardiovascular risk factors, in patients with CAC=0 on their baseline scan.62 As a fraction of total coronary plaque, non-calcified plaque is more prevalent in younger populations,63 particularly in patients presenting with ACS in the absence of stable CAD.64 Specific cardiovascular risk factors, including diabetes and hyperlipidaemia, are also linked with higher burden of non-calcified plaque and are critical to consider in younger patients.61,65,66
The importance of age in CAC score interpretation is supported by literature findings.67 An early prospective study by Knez et al. evaluated 2,115 symptomatic patients with chest pain referred for formal angiography (mean age 62 years). Among 326 patients with CAC=0, none had significant CAD defined as ≥50% luminal stenosis.68 Among 1,247 patients with any degree of angiographically confirmed stenosis, only eight had CAC=0 (0.6%), seven of whom were under age 45 years.68 While the rate of subclinical stenosis in symptomatic adults over age 45 years with CAC=0 was only ~0.05%, the rate in adults under 45 years was ~2-5%, up to 100-fold higher.68 In another notable study, Chang et al. reported a prospective series of 1,049 patients (median age 48 years) presenting to an ED with acute chest pain and suspicion for ACS. Obstructive CAD was found in 17 of 76 patients with CAC=0 (NPV=77.6%), four of whom suffered a cardiovascular event within 30 days.58 The authors found that patients under 50 years were 70% more likely to have obstructive CAD with CAC=0.58 Cademartiri and colleagues reported ≥50% stenosis in 14.6% of 279 patients selected on the basis of suspected CAD, despite CAC=0.49 Although these numbers were driven by high pre-test probability in terms of symptoms and risk profile (including known ischaemic changes on stress testing in many patients), it bears mention that mean age across this cohort was only 48 years. Akram et al. also published a series of 210 patients referred for CAC scoring and CCTA, and reported >70% stenosis in four of 49 (8.2%) symptomatic patients on CCTA, despite CAC=0; three of these four patients were under age 45 years.50
Recently, the age-dependent relationship between CAC scores and risk of obstructive CAD has been rigorously examined in a well-conducted study by Mortensen et al.60 Mortensen and colleagues examined data from 23,759 patients aged 18 years or older from the Western Denmark Heart Registry. Among 13,496 patients with CAC=0 (57%), 725 patients (5.7%) had obstructive CAD on CCTA, corresponding to an NPV of 94.3%. Prevalence of CAC=0, however, varied markedly with age. CAC=0 was observed in 93% (1,278 of 1,372) of patients under 40 years, compared with only 51% (11,493 of 22,387) of patients over 40 years. Younger patients with obstructive CAD were much more likely to have non-calcified plaque. Among patients with obstructive CAD, CAC=0 was observed in 58% (39/68) of patients <40 years compared with only 14% (686/4,975) of patients >40 years, and 5% (52/964) of patients >70 years. Moreover, the diagnostic value of CAC=0 in reclassifying obstructive CAD risk increased steadily with age. After adjusting for age, sex, smoking, diabetes, and symptom characteristics, the risk-adjusted diagnostic likelihood ratio of obstructive CAD for CAC=0 ranged from 0.68 (32% lower CAD likelihood) in patients aged 18–39 years down to 0.18 (82% lower CAD likelihood) for patients >70 years. These findings corroborate the existing literature and demonstrate the age-dependent value of a zero CAC score.
Illustrative case
A 31-year-old man presented to our hospital with intermittent left-sided chest pain and radiation to his left arm. Associated symptoms included shortness of breath and diaphoresis. He described experiencing similar symptoms in recent months. Medical history was pertinent for obesity, uncontrolled diabetes, and hypertension. Laboratory values revealed a glycosylated haemoglobin (HbA1c) of 14.1% and lipid profile significant for total cholesterol of 364 mg/dL, high-density lipoprotein (HDL) 24 mg/dL, and triglycerides of 1,146 mg/dL. ECG showed normal sinus rhythm without evidence of ischaemia, infarction, or hypertrophy. Echocardiogram was structurally normal, with normal ejection fraction and wall motion. Troponin I levels were intermediate (peak 0.24 ng/mL) and he was assessed as having intermediate-risk chest pain.
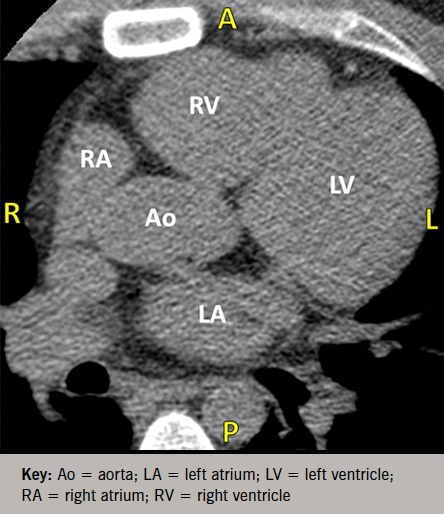
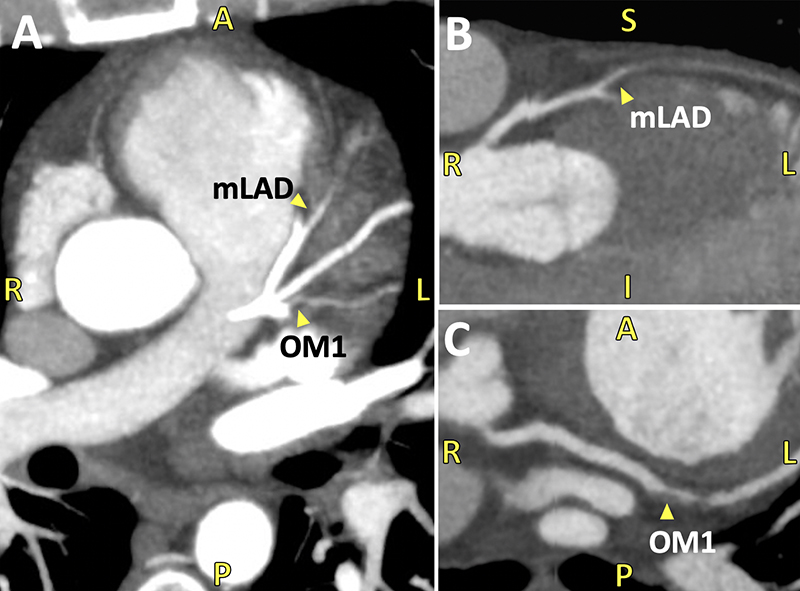
As part of a protocol at our hospital, patients with chest pain and an intermediate risk profile proceed directly to CCTA to exclude obstruction. The patient had a CAC score of zero (figure 1), but CCTA revealed severe two-vessel disease with total occlusion of the mid-left anterior descending (LAD) and proximal first obtuse marginal (OM1) arteries (figure 2). Coronary angiography confirmed complete mid-LAD occlusion with reconstituted distal flow supplied via right-to-left collaterals, and 80% proximal stenosis of the OM1. He underwent successful revascularisation with two-vessel coronary artery bypass grafting and was discharged in stable condition.
Conclusion
In this paper, we review the strong evidence supporting CAC as a diagnostic and prognostic marker of ASCVD. We consider the diagnostic implications of a zero CAC score, with attention to its age-dependent limitations. In older adults, CAC=0 is a validated biomarker portending low risk of cardiovascular events.55 However, as reflected in our illustrative case, CAC=0 does not reliably exclude obstructive CAD in younger patients, who are known to have a higher burden of non-calcified plaque. Here, CCTA is irreplaceable for guiding decision-making in both emergency and outpatient settings. When the diagnosis is in question, adults under 40 years with chest pain and an intermediate risk profile should proceed directly to CCTA. Future work will further expand our understanding of CAC scores across the demographic spectrum, facilitating optimal integration of this tool to best evaluate risk and guide interventions in the individual patient.
Key messages
- The coronary artery calcium (CAC) score is a powerful, well-validated biomarker that reliably predicts risk burden in atherosclerotic cardiovascular disease
- In older adults, CAC score of zero reliably down-stratifies risk of obstructive coronary artery disease (CAD)
- In adults under 40 years, zero CAC score does not reliably exclude obstructive CAD due to higher prevalence of non-calcified plaque
- Younger patients with chest pain and risk factors raising suspicion for CAD should proceed directly to coronary computed tomography angiography (CCTA)
Conflicts of Interest
MJB is a consultant for General Electric. JP, SL, SJL, SKR: none declared.
Funding
This work was supported by funding from the National Heart, Lung, and Blood Institute (NHLBI) of the United States National Institutes of Health (NIH) under award number NIHR01HL146666.
Patient consent
Informed patient consent was obtained for the clinical case included in this manuscript.
References
1. Nasir K, Cainzos-Achirica M. Role of coronary artery calcium score in the primary prevention of cardiovascular disease. BMJ 2021;373:n776. https://doi.org/10.1136/bmj.n776
2. Gagel AC, Blumenthal RS, Cainzos-Achirica M. The ever-growing role of coronary artery calcium in primary prevention. American College of Cardiology: Latest in Cardiology. Published online 21 June 2021. Available from: https://www.acc.org/latest-in-cardiology/articles/2021/06/21/13/05/the-ever-growing-role-of-cac-in-primary-prevention
3. Mach F, Baigent C, Catapano AL et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J 2019;41:111–88. https://doi.org/10.1093/eurheartj/ehz455
4. Grundy SM, Stone NJ, Bailey AL et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;139:e1082–e1143. https://doi.org/10.1161/CIR.0000000000000698
5. Arnett DK, Blumenthal RS, Albert MA et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;140:e596–e646. https://doi.org/10.1161/CIR.0000000000000725
6. Piepoli MF, Abreu A, Albus C et al. Update on cardiovascular prevention in clinical practice: a position paper of the European Association of Preventive Cardiology of the European Society of Cardiology. Eur J Prev Cardiol 2020;27:181–205. https://doi.org/10.1177/2047487319893035
7. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827–32. https://doi.org/10.1016/0735-1097(90)90282-T
8. Rumberger JA, Brundage BH, Rader DJ, Kondos G. Electron beam computed tomographic coronary calcium scanning: a review and guidelines for use in asymptomatic persons. Mayo Clin Proc 1999;74:243–52. https://doi.org/10.4065/74.3.243
9. Taylor AJ, Bindeman J, Feuerstein I, Cao F, Brazaitis M, O’Malley PG. Coronary calcium independently predicts incident premature coronary heart disease over measured cardiovascular risk factors: mean three-year outcomes in the Prospective Army Coronary Calcium (PACC) project. J Am Coll Cardiol 2005;46:807–14. https://doi.org/10.1016/j.jacc.2005.05.049
10. Vliegenthart R, Oudkerk M, Hofman A et al. Coronary calcification improves cardiovascular risk prediction in the elderly. Circulation 2005;112:572–7. https://doi.org/10.1161/CIRCULATIONAHA.104.488916
11. LaMonte MJ, FitzGerald SJ, Church TS et al. Coronary artery calcium score and coronary heart disease events in a large cohort of asymptomatic men and women. Am J Epidemiol 2005;162:421–9. https://doi.org/10.1093/aje/kwi228
12. Arad Y, Goodman KJ, Roth M, Newstein D, Guerci AD. Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study. J Am Coll Cardiol 2005;46:158–65. https://doi.org/10.1016/j.jacc.2005.02.088
13. Detrano R, Guerci AD, Carr JJ et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med 2008;358:1336–45. https://doi.org/10.1056/NEJMoa072100
14. Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA 2004;291:210–15. https://doi.org/10.1001/jama.291.2.210
15. Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97:1837–47. https://doi.org/10.1161/01.CIR.97.18.1837
16. Hoffmann U, Massaro JM, D’Agostino RB Sr., Kathiresan S, Fox CS, O’Donnell CJ. Cardiovascular event prediction and risk reclassification by coronary, aortic, and valvular calcification in the Framingham Heart Study. J Am Heart Assoc 2016;5:e003144. https://doi.org/10.1161/JAHA.115.003144
17. Shareghi S, Ahmadi N, Young E, Gopal A, Liu ST, Budoff MJ. Prognostic significance of zero coronary calcium scores on cardiac computed tomography. J Cardiovasc Comput Tomogr 2007;1:155–9. https://doi.org/10.1016/j.jcct.2007.10.001
18. Raggi P, Callister TQ, Cooil B et al. Identification of patients at increased risk of first unheralded acute myocardial infarction by electron-beam computed tomography. Circulation 2000;101:850–5. https://doi.org/10.1161/01.CIR.101.8.850
19. Erbel R, Mohlenkamp S, Moebus S et al. Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis: the Heinz Nixdorf Recall study. J Am Coll Cardiol 2010;56:1397–406. https://doi.org/10.1016/j.jacc.2010.06.030
20. Budoff MJ, Shaw LJ, Liu ST et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol 2007;49:1860–70. https://doi.org/10.1016/j.jacc.2006.10.079
21. Blaha M, Budoff MJ, Shaw LJ et al. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging 2009;2:692–700. https://doi.org/10.1016/j.jcmg.2009.03.009
22. Blaha MJ, Cainzos-Achirica M, Dardari Z et al. All-cause and cause-specific mortality in individuals with zero and minimal coronary artery calcium: a long-term, competing risk analysis in the Coronary Artery Calcium Consortium. Atherosclerosis 2020;294:72–9. https://doi.org/10.1016/j.atherosclerosis.2019.11.008
23. Blaha MJ, Cainzos-Achirica M, Greenland P et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2016;133:849–58. https://doi.org/10.1161/CIRCULATIONAHA.115.018524
24. Nasir K, Bittencourt MS, Blaha MJ et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association cholesterol management guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2015;66:1657–68. https://doi.org/10.1016/j.jacc.2015.07.066
25. Stone NJ, Robinson JG, Lichtenstein AH et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63(25 Pt B):2889–934. https://doi.org/10.1161/01.cir.0000437738.63853.7a
26. Valenti V, Hartaigh BO, Heo R et al. A 15-year warranty period for asymptomatic individuals without coronary artery calcium: a prospective follow-up of 9,715 individuals. JACC Cardiovasc Imaging 2015;8:900–09. https://doi.org/10.1016/j.jcmg.2015.01.025
27. Knuuti J, Wijns W, Saraste A et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020;41:407–77. https://doi.org/10.1093/eurheartj/ehz425
28. Hussain A, Ballantyne CM, Nambi V. Zero coronary artery calcium score: desirable, but enough? Circulation 2020;142:917–19. https://doi.org/10.1161/CIRCULATIONAHA.119.045026
29. Fernandez-Friera L, Garcia-Alvarez A, Guzman G, Garcia MJ. Coronary CT and the coronary calcium score, the future of ED risk stratification? Curr Cardiol Rev 2012;8:86–97. https://doi.org/10.2174/157340312801784989
30. Budoff MJ, Dowe D, Jollis JG et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol 2008;52:1724–32. https://doi.org/10.1016/j.jacc.2008.07.031
31. Neglia D, Rovai D, Caselli C et al. Detection of significant coronary artery disease by noninvasive anatomical and functional imaging. Circ Cardiovasc Imaging 2015;8:e002179. https://doi.org/10.1161/CIRCIMAGING.114.002179
32. Budoff MJ, Li D, Kazerooni EA, Thomas GS, Mieres JH, Shaw LJ. Diagnostic accuracy of noninvasive 64-row computed tomographic coronary angiography (CCTA) compared with myocardial perfusion imaging (MPI): the PICTURE study, a prospective multicenter trial. Acad Radiol 2017;24:22–9. https://doi.org/10.1016/j.acra.2016.09.008
33. Hoffmann U, Truong QA, Schoenfeld DA et al. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med 2012;367:299–308. https://doi.org/10.1056/NEJMoa1201161
34. Budoff MJ, Mayrhofer T, Ferencik M et al. Prognostic value of coronary artery calcium in the PROMISE study (Prospective Multicenter Imaging Study for Evaluation of Chest Pain). Circulation 2017;136:1993–2005. https://doi.org/10.1161/CIRCULATIONAHA.117.030578
35. Hoffmann U, Ferencik M, Udelson JE et al. Prognostic value of noninvasive cardiovascular testing in patients with stable chest pain: insights from the PROMISE trial (Prospective Multicenter Imaging Study for Evaluation of Chest Pain). Circulation 2017;135:2320–32. https://doi.org/10.1161/CIRCULATIONAHA.116.024360
36. Investigators S-H, Newby DE, Adamson PD et al. Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med 2018;379:924–33. https://doi.org/10.1056/NEJMoa1805971
37. Abuzaid A, Saad M, Addoumieh A et al. Coronary artery calcium score and risk of cardiovascular events without established coronary artery disease: a systemic review and meta-analysis. Coron Artery Dis 2021;32:317–28. https://doi.org/10.1097/MCA.0000000000000974
38. Mahmood T, Shapiro MD. Coronary artery calcium testing in low-intermediate risk symptomatic patients with suspected coronary artery disease: an effective gatekeeper to further testing? PloS One 2020;15:e0240539. https://doi.org/10.1371/journal.pone.0240539
39. Villines TC, Hulten EA, Shaw LJ et al. Prevalence and severity of coronary artery disease and adverse events among symptomatic patients with coronary artery calcification scores of zero undergoing coronary computed tomography angiography: results from the CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter) registry. J Am Coll Cardiol 2011;58:2533–40. https://doi.org/10.1016/j.jacc.2011.10.851
40. Lubbers M, Dedic A, Coenen A et al. Calcium imaging and selective computed tomography angiography in comparison to functional testing for suspected coronary artery disease: the multicentre, randomized CRESCENT trial. Eur Heart J 2016;37:1232–43. https://doi.org/10.1093/eurheartj/ehv700
41. Lubbers M, Coenen A, Kofflard M et al. Comprehensive cardiac CT with myocardial perfusion imaging versus functional testing in suspected coronary artery disease: the multicenter, randomized CRESCENT-II trial. JACC Cardiovasc Imaging 2018;11:1625–36. https://doi.org/10.1016/j.jcmg.2017.10.010
42. Williams MC, Moss AJ, Dweck M et al. Coronary artery plaque characteristics associated with adverse outcomes in the SCOT-HEART study. J Am Coll Cardiol 2019;73:291–301. https://doi.org/10.1016/j.jacc.2018.10.066
43. Wang X, Le EPV, Rajani NK et al. A zero coronary artery calcium score in patients with stable chest pain is associated with a good prognosis, despite risk of non-calcified plaques. Open Heart 2019;6:e000945. https://doi.org/10.1136/openhrt-2018-000945
44. Laudon DA, Behrenbeck TR, Wood CM et al. Computed tomographic coronary artery calcium assessment for evaluating chest pain in the emergency department: long-term outcome of a prospective blind study. Mayo Clin Proc 2010;85:314–22. https://doi.org/10.4065/mcp.2009.0620
45. McLaughlin VV, Balogh T, Rich S. Utility of electron beam computed tomography to stratify patients presenting to the emergency room with chest pain. Am J Cardiol 1999;84:327–8, A8. https://doi.org/10.1016/S0002-9149(99)00286-6
46. Georgiou D, Budoff MJ, Kaufer E, Kennedy JM, Lu B, Brundage BH. Screening patients with chest pain in the emergency department using electron beam tomography: a follow-up study. J Am Coll Cardiol 2001;38:105–10. https://doi.org/10.1016/S0735-1097(01)01364-X
47. Bittner DO, Takx RAP, Staziaki PV et al. Identification of coronary artery calcification can optimize risk stratification in patients with acute chest pain. Int J Cardiol 2017;249:473–8. https://doi.org/10.1016/j.ijcard.2017.06.119
48. Mittal TK, Pottle A, Nicol E et al. Prevalence of obstructive coronary artery disease and prognosis in patients with stable symptoms and a zero-coronary calcium score. Eur Heart J Cardiovasc Imaging 2017;18:922–9. https://doi.org/10.1093/ehjci/jex037
49. Cademartiri F, Maffei E, Palumbo A et al. Diagnostic accuracy of computed tomography coronary angiography in patients with a zero calcium score. Eur Radiol 2010;20:81–7. https://doi.org/10.1007/s00330-009-1529-9
50. Akram K, O’Donnell RE, King S, Superko HR, Agatston A, Voros S. Influence of symptomatic status on the prevalence of obstructive coronary artery disease in patients with zero calcium score. Atherosclerosis 2009;203:533–7. https://doi.org/10.1016/j.atherosclerosis.2008.07.008
51. Gottlieb I, Miller JM, Arbab-Zadeh A et al. The absence of coronary calcification does not exclude obstructive coronary artery disease or the need for revascularization in patients referred for conventional coronary angiography. J Am Coll Cardiol 2010;55:627–34. https://doi.org/10.1016/j.jacc.2009.07.072
52. Haberl R, Tittus J, Bohme E et al. Multislice spiral computed tomographic angiography of coronary arteries in patients with suspected coronary artery disease: an effective filter before catheter angiography? Am Heart J 2005;149:1112–19. https://doi.org/10.1016/j.ahj.2005.02.048
53. Henneman MM, Schuijf JD, Pundziute G et al. Noninvasive evaluation with multislice computed tomography in suspected acute coronary syndrome: plaque morphology on multislice computed tomography versus coronary calcium score. J Am Coll Cardiol 2008;52:216–22. https://doi.org/10.1016/j.jacc.2008.04.012
54. Rubinshtein R, Gaspar T, Halon DA, Goldstein J, Peled N, Lewis BS. Prevalence and extent of obstructive coronary artery disease in patients with zero or low calcium score undergoing 64-slice cardiac multidetector computed tomography for evaluation of a chest pain syndrome. Am J Cardiol 2007;99:472–5. https://doi.org/10.1016/j.amjcard.2006.08.060
55. Sarwar A, Shaw LJ, Shapiro MD et al. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging 2009;2:675–88. https://doi.org/10.1016/j.jcmg.2008.12.031
56. Dzaye O, Dardari ZA, Cainzos-Achirica M et al. Warranty period of a calcium score of zero: comprehensive analysis from MESA. JACC Cardiovasc Imaging 2021;14:990–1002. https://doi.org/10.1016/j.jcmg.2020.06.048
57. Otsuka F, Sakakura K, Yahagi K, Joner M, Virmani R. Has our understanding of calcification in human coronary atherosclerosis progressed? Arterioscler Thromb Vasc Biol 2014;34:724–36. https://doi.org/10.1161/ATVBAHA.113.302642
58. Chang AM, Le J, Matsuura AC, Litt HI, Hollander JE. Does coronary artery calcium scoring add to the predictive value of coronary computed tomography angiography for adverse cardiovascular events in low-risk chest pain patients? Acad Emerg Med 2011;18:1065–71. https://doi.org/10.1111/j.1553-2712.2011.01173.x
59. Alexopoulos N, Raggi P. Calcification in atherosclerosis. Nat Rev Cardiol 2009;6:681–8. https://doi.org/10.1038/nrcardio.2009.165
60. Mortensen MB, Gaur S, Frimmer A et al. Association of age with the diagnostic value of coronary artery calcium score for ruling out coronary stenosis in symptomatic patients. JAMA Cardiology 2022;7:36–44. https://doi.org/10.1001/jamacardio.2021.4406
61. Kral BG, Becker LC, Vaidya D et al. Noncalcified coronary plaque volumes in healthy people with a family history of early onset coronary artery disease. Circ Cardiovasc Imaging 2014;7:446–53. https://doi.org/10.1161/CIRCIMAGING.113.000980
62. Budoff MJ, Young R, Lopez VA et al. Progression of coronary calcium and incident coronary heart disease events: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2013;61:1231–9. https://doi.org/10.1016/j.jacc.2012.12.035
63. Mortensen MB, Fuster V, Muntendam P et al. A simple disease-guided approach to personalize ACC/AHA-recommended statin allocation in elderly people: the BioImage study. J Am Coll Cardiol 2016;68:881–91. https://doi.org/10.1016/j.jacc.2016.05.084
64. Pundziute G, Schuijf JD, Jukema JW et al. Evaluation of plaque characteristics in acute coronary syndromes: non-invasive assessment with multi-slice computed tomography and invasive evaluation with intravascular ultrasound radiofrequency data analysis. Eur Heart J 2008;29:2373–81. https://doi.org/10.1093/eurheartj/ehn356
65. Scholte AJ, Schuijf JD, Kharagjitsingh AV et al. Prevalence of coronary artery disease and plaque morphology assessed by multi-slice computed tomography coronary angiography and calcium scoring in asymptomatic patients with type 2 diabetes. Heart 2008;94:290–5. https://doi.org/10.1136/hrt.2007.121921
66. Santos RD. Calcified and noncalcified coronary plaques and atherosclerotic cardiovascular events in patients with severe hypercholesterolemia-moving forward with risk stratification and therapy. JAMA Netw Open 2022;5:e2148147. https://doi.org/10.1001/jamanetworkopen.2021.48147
67. Akram K, Voros S. Influence of symptoms and age on the predictive value of coronary artery calcium scanning. J Am Coll Cardiol 2008;52:2214; author reply 2214. https://doi.org/10.1016/j.jacc.2008.07.070
68. Knez A, Becker A, Leber A et al. Relation of coronary calcium scores by electron beam tomography to obstructive disease in 2,115 symptomatic patients. Am J Cardiol 2004;93:1150–2. https://doi.org/10.1016/j.amjcard.2004.01.044
69. Paixao AR, Ayers CR, El Sabbagh A et al. Coronary artery calcium improves risk classification in younger populations. JACC Cardiovasc Imaging 2015;8:1285–93. https://doi.org/10.1016/j.jcmg.2015.06.015
70. Sung JH, Yeboah J, Lee JE et al. Diagnostic value of coronary artery calcium score for cardiovascular disease in African Americans: the Jackson Heart Study. Br J Med Med Res 2016;11:BJMMR/2016/21449. https://doi.org/10.9734/BJMMR/2016/21449
71. Carr JJ, Jacobs DR Jr., Terry JG et al. Association of coronary artery calcium in adults aged 32 to 46 years with incident coronary heart disease and death. JAMA Cardiol 2017;2:391–9. https://doi.org/10.1001/jamacardio.2016.5493
72. Grandhi GR, Mirbolouk M, Dardari ZA et al. Interplay of coronary artery calcium and risk factors for predicting CVD/CHD mortality: the CAC consortium. JACC Cardiovasc Imaging 2020;13:1175–86. https://doi.org/10.1016/j.jcmg.2019.08.024
73. Kim YJ, Hur J, Lee HJ et al. Meaning of zero coronary calcium score in symptomatic patients referred for coronary computed tomographic angiography. Eur Heart J Cardiovasc Imaging 2012;13:776–85. https://doi.org/10.1093/ehjci/jes060
74. Hulten E, Bittencourt MS, Ghoshhajra B et al. Incremental prognostic value of coronary artery calcium score versus CT angiography among symptomatic patients without known coronary artery disease. Atherosclerosis 2014;233:190–5. https://doi.org/10.1016/j.atherosclerosis.2013.12.029
75. Mitchell JD, Fergestrom N, Gage BF et al. Impact of statins on cardiovascular outcomes following coronary artery calcium scoring. J Am Coll Cardiol 2018;72:3233–42. https://doi.org/10.1016/j.jacc.2018.09.051
