Dual antiplatelet therapy is recommended for secondary prevention of ischaemic events in coronary artery disease. Some patients, who may be at high bleed risk if other factors are present, should be considered for gastroprotection. In our survey, we assessed whether gastroprotection was prescribed for hospital inpatients, especially high-risk patients, who were receiving dual antiplatelet therapy at discharge, and the type of gastroprotection prescribed. We found that over 13 months, a total of 1,693 patient episodes were prescribed dual antiplatelet therapy at discharge, of which 71% also received gastroprotection. Of the patient episodes who were not prescribed gastroprotection, 46% (223/483) met the criterion of age as a risk factor for gastroprotection. A further 30 episodes met other risk criteria of certain concomitant drugs or prior comorbidity. There is a need among clinicians and pharmacy teams within the hospital for recognition and management of this opportunity to improve the care of these patients.
Introduction
Dual antiplatelet therapy (DAPT), a combination of aspirin and either clopidogrel, prasugrel or ticagrelor, is recommended for secondary prevention of ischaemic events in coronary artery disease in both patients managed medically and those undergoing percutaneous coronary intervention (PCI). Patients taking DAPT may be at high bleed risk if other factors are present, such as older age, kidney and/or liver disease, active cancer, anaemia, low platelet count, previous stroke, prior bleeding, recent trauma or surgery, and use of oral anticoagulants and/or non-steroidal anti-inflammatory drugs (NSAIDs).1 Gastrointestinal (GI) bleeding is a particularly serious DAPT-related complication, and gastroprotection with a proton-pump inhibitor (PPI) or H2-receptor antagonist should be considered for certain groups of patients, though guidance on which groups should receive gastroprotection is variable.
Box 1. Risk factors for gastrointestinal (GI) bleed
|
European guidelines recommend a PPI for all patients, whereas American guidance is more targeted at high-risk patients.2,3 As a concise summary to be followed in England, National Institute for Health and Care Excellence (NICE) Clinical Knowledge Summaries identify people at high risk of GI adverse effects with antiplatelet treatment if the risk factors in box 1 are present.4 Gastroprotection reduces this bleeding risk by 70% or more, and COGENT (Clopidogrel and the Optimization of Gastrointestinal Events Trial) showed that the addition of omeprazole 20 mg daily to aspirin–clopidogrel dual therapy in patients with a median age of 69 years reduced overt gastroduodenal bleeding from an absolute 0.6% down to 0.1% at 180 days after randomisation.5 A post-hoc analysis of the COGENT trial evaluated the safety and efficacy of PPI therapy in the post-acute coronary syndrome (ACS) group. This study, in a cohort of high-risk patients, showed significant benefit with bleeding reduced from 1.2% to 0.24% at 180-day follow-up.6 Others have shown that PPIs are superior to H2-receptor antagonists for gastroprotection in patients on DAPT, though this review included trials using low and high doses of each class of drug.7
Some patients (e.g. those with existing atrial fibrillation or those who develop atrial fibrillation after PCI, coronary artery bypass graft or ACS) are prescribed triple therapy – an anticoagulant in addition to DAPT – which further increases the risk of bleeding. These patients should automatically be considered for gastroprotection. For instance, our acute hospital trust policy notes that compared with oral anticoagulation therapy alone, the addition of DAPT to oral anticoagulation therapy results in at least a two- to threefold increase in bleeding complications, and that routine use of PPIs is recommended.8 Though not mentioned in the policy, current practice recognises there are cautions when considering use of a PPI, such as electrolyte abnormalities.
However, various studies from across the globe have reported that patients on DAPT and at high risk of bleeding have not received concomitant gastroprotection. One Danish study examined 46,301 patients on DAPT after a myocardial infarction.9 Only 35% of patients at higher risk of upper GI bleeding received the recommended treatment with a PPI to reduce bleeding risk related to PCI based on the 2015 European Society of Cardiology (ESC) guideline criteria, which are broader than those factors described in box 1, including a lower age threshold of ≥65 years.10 An American study from 2011, using an age risk factor ≥75 years, noted that in a sample of 250 hospital patients the use of GI prophylaxis was appropriate in only 48% of patients.11 A UK study from 2008 found that less than half of 370 ACS patients at high bleeding risk taking DAPT were provided with GI prophylaxis and, in particular, of the ≥75 years cohort only 50% received such GI prophylaxis.12 Interestingly, all the above studies apparently examined only the presence or absence of concomitant GI prophylaxis and did not look at the dose prescribed.
As well as the association between DAPT and increased GI bleeding,13 adverse drug reactions due to antiplatelets have been associated with hospital admission, including preventable admissions.14-16 One specific area of medicines safety – measuring the number of patients aged 18 years and over currently prescribed aspirin and another antiplatelet without a gastroprotective medicine – was identified in the Investment and Impact Fund (IIF) 2020/21 for primary care in England.17 This IIF, which is a financial incentive scheme, supports primary care networks in England to deliver high-quality care to their population, as well as supporting the delivery of priority objectives in the National Health Service (NHS) Long Term Plan. Primary care networks consist of GP practices working together with community, mental health, social care, pharmacy, hospital and voluntary services in their local areas in groups of practices. The indicators in the IIF domain of delivering better outcomes for patients on medication focused on improved prescribing to support a reduction in medicines-related harm, and the IIF metric for the number of patients on DAPT and a gastroprotective is reported on the NHS Business Services Authority medicines safety dashboard at various levels including practice, and primary care network.18
Aims and objectives
The main aim of this review was to assess whether gastroprotection was prescribed for hospital inpatients, especially high-risk patients, who were receiving DAPT at discharge, and the type of gastroprotection prescribed.
Method
Study design and setting
This was a descriptive, retrospective study in a 750-bed acute hospital in the south-west of England serving a population of 450,000, which doubles over the summer holiday period.
Data collection and processing
Patient episodes involving prescription of DAPT (aspirin plus concomitant use of clopidogrel, ticagrelor or prasugrel) upon discharge between April 2020 and April 2021 were included in the data extraction from the hospital e-prescribing system (Wellsky International, Basildon, UK). The electronic prescribing records of the identified patients were also searched for co-prescription of gastroprotection, either PPI or H2 antagonist, and if there was a documented contraindication to gastroprotection. This prescribing database was also analysed to ascertain if patients on DAPT should have been on gastroprotection using risk factors of age 71 years and over, some concomitant medication (selective serotonin reuptake inhibitor [SSRI], NSAID, prednisolone, nicorandil or an anticoagulant), and also if patients were on triple therapy (dual antiplatelets and an anticoagulant). A list of diagnostic codes was generated for those patients not prescribed gastroprotection identifying the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10) administrative code, if present, in primary or secondary position in hospital episode statistics for the episode of care. This identified patients with the following diagnostic codes: gastrointestinal haemorrhage, unspecified (K92.2); gastric ulcer, unspecified as acute or chronic, without haemorrhage or perforation (K25.9); personal history of diseases of the digestive system (Z87.1). Demographic details were also recorded, as were any potential biochemical abnormalities that might influence the use of a PPI as gastroprotection. Data were entered into Microsoft Excel for analysis.
Results
Over this 13-month period there was a total of 1,693 patient episodes (mean age 72 years, 63% male) prescribed DAPT at discharge, of which 1,210 (71%) also received gastroprotection (figure 1). Of the 483 patient episodes who were not prescribed gastroprotection, 223 (46%) met the criterion of age as a risk factor for gastroprotection.
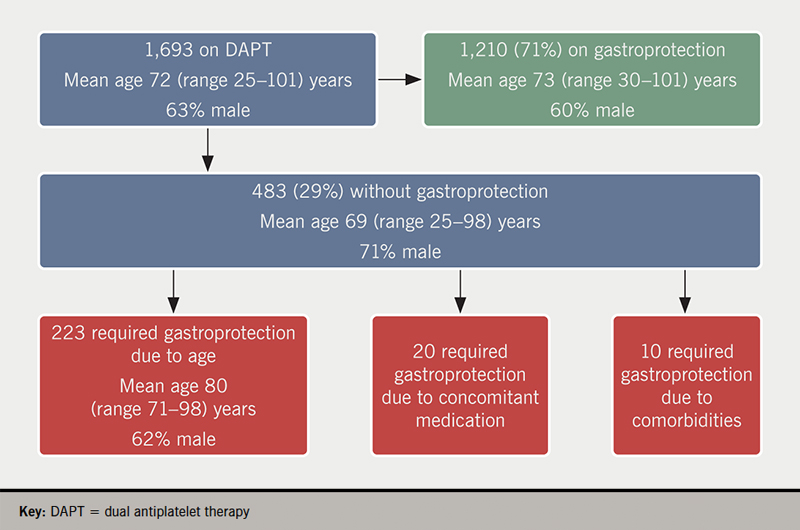
There were 20 episodes (mean age 61 years, range 38–69, 80% male) of patients aged under 71 years not on gastroprotection but receiving a SSRI, NSAID, prednisolone, nicorandil or an anticoagulant. In one of these episodes a patient received nicorandil and an anticoagulant in addition to DAPT and so was very much at high bleeding risk. There were a further 10 patient spells without gastroprotection but with an ICD code of previous history of GI ulcer/bleed, and all these were younger than 71 years of age. Hence, we identified that gastroprotection was potentially missing in a total of 15% (n=253) of DAPT patient episodes. Of those 253 episodes where we would have expected patients to be prescribed gastroprotection but were not, we observed 47 unique episodes where a patient had either an abnormal value, as defined by our clinical chemistry department, of a serum magnesium level <0.7 mmol/L or a serum sodium level <133 mmol/L during that spell. In these patients, our clinical staff consider that use of a PPI, though desirable, is inappropriate. The remaining 206 episodes had no such abnormal electrolyte contraindication.
Of those 1,210 episodes of patients receiving gastroprotection, a PPI was prescribed in 1,171 instances. There were 49% (n=587) prescriptions for lansoprazole, 48% (n=564) omeprazole (of which 34 were for the 10 mg strength), and 10 each of esomeprazole, and rabeprazole. A H2 antagonist was prescribed in 39 instances, of which 72% (n=28) were for famotidine, and 11 ranitidine. For the 28 patient episodes of famotidine prescribed as gastroprotection, 13 of these instances were recorded as due to the patient being unable to receive a PPI due to low sodium and/or low magnesium.
Discussion
In this retrospective observational study, we found that gastroprotection was not prescribed during the hospital admission for 29% (n=483) of 1,693 patient episodes receiving DAPT. Approximately half (253) of those who did not receive gastroprotection, equivalent to 15% of the total cohort of episodes, were classified as at high risk for GI bleed based on age, or a selection of concomitant medication, or specific limited comorbidities. In one English study, GI bleeding was identified as the single greatest cause of hospital admission or death due to adverse drug reactions, largely caused by prescribed antithrombotics.19 A recent systematic review and meta-analysis found that the use of PPIs was associated with a reduced risk of GI bleeding in patients treated with DAPT after PCI or ACS.20 Hence, consideration of gastroprotection, certainly for high-risk patients, is an important aspect of the pharmaceutical care of these patients.
The inclusion in the English NHS IIF for general practice potentially has implications for hospitals.17 This IIF metric measures the percentage of patients aged 18 years or over prescribed aspirin and another antiplatelet in the three months to 1 April 2022, who in the three months to 1 April 2023 were either (i) no longer prescribed aspirin and/or no longer prescribed an antiplatelet or (ii) prescribed a gastroprotective in addition to both aspirin and another antiplatelet. There are thresholds for payment to general practice of 75% as the lower threshold and 90% as the upper threshold. Although this IIF metric does not directly apply to what is happening in English hospitals, our observed value of 71% of our cohort of patient episodes on DAPT receiving gastroprotection may attract scrutiny from primary care wanting to see a higher proportion of patients discharged on gastroprotection. This may be especially relevant as primary care prescribing across Cornwall is shown to be higher than the England average for having patients on DAPT but not receiving gastroprotection, with Cornwall having approximately 4,000 patients in the final quarter of 2020/2021 compared with an expected value of 3,000.18
Though antiplatelets are listed as high-risk medicines and seen as a candidate for prioritisation for a medication review in hospitals, it is not clear from the literature if this is solely because of the risk of GI bleeding and the need for gastroprotection, or other reasons, such as appropriate indication or duration for treatment.21-23 Our age threshold for increased risk was 71 years and over, and we note that other studies use a different age threshold for high-risk prescribing of DAPT.24,25 For instance, a Canadian study of cardiology outpatients found that 57.1% (n=68) of 119 patients aged over 60 years on DAPT were receiving a PPI.26
When considering newly initiated gastroprotection when one of the antiplatelet agents is clopidogrel, the choice of PPI may be a consideration, though up to now our trust has not been overly concerned about the theoretical interaction between clopidogrel and omeprazole.27 However, the Care Quality Commission, which monitor, inspect and regulate general practice services to make sure they meet fundamental standards of quality and safety, now scrutinise how general practitioners manage this potential interaction.28 Therefore, it is expected to become more of an issue for hospitals discharging patients on this combination. In fact, of the 574 patient spells in our study when omeprazole or esomeprazole was co-prescribed, clopidogrel was the antiplatelet used in combination with aspirin in 261/574 (45%) of cases.
PPIs are not without risk and are associated with slight increased risk of bone fractures and pneumonia, and an association with vitamin B deficiency, and with Clostridium difficile infection in hospitalised patients. PPIs are also known to have adverse effects, such as low sodium and low magnesium,29 which may be a reason for considering a H2 antagonist. The European guidelines advise that impaired magnesium absorption with PPIs has been reported only from studies in which patients had received a PPI for at least one year.2 However, a recent review of PPI adverse effects found that most putative adverse outcomes associated with PPI use may not be supported by high-quality evidence and are likely to have been affected by underlying confounding factors.30 We found that in 19% (47/243) instances, the reason for not prescribing a PPI appeared to be low sodium or low magnesium.
Strengths and limitations
This is a large cross-sectional study that collected data on all patients with prescribed DAPT over a 13-month period. There are, however, limitations. First, it was conducted in a single centre in England; this might restrict the generalisability of our findings. Second, the retrospective nature of this may introduce bias or other uncertainties. Third, we did not ascertain the indication for DAPT nor confirm if the PPI or H2 antagonist was actually for gastroprotection or another indication, nor if patients came in on these drugs as opposed to being started during their admission. Fourth, we did not check risk factors for gastroprotection other than age, selected concomitantly prescribed drugs, and selected comorbidities. Finally, we did not check if those not prescribed gastroprotection had significant contraindications other than electrolyte abnormalities.
Conclusion
We identified that 15% of patients on DAPT were at high risk for GI bleed and, yet, did not receive appropriate GI prophylaxis. There is a need among clinicians and pharmacy teams within the hospital for recognition and management of this opportunity to improve the care of these patients. This will include updating local guidelines to incorporate the recommendation on gastroprotection in patients on dual antiplatelets and at risk of GI bleeding.
Key messages
- Dual antiplatelet therapy (DAPT) is a risk factor for gastrointestinal bleeding and concomitant gastroprotection is recommended to reduce the risk of bleeding if there are various patient risk factors
- We assessed whether gastroprotection was prescribed for hospital inpatients, especially high-risk patients, who were receiving DAPT at discharge, and the type of gastroprotection prescribed
- Over 13 months, a total of 1,693 patient episodes were prescribed DAPT at discharge, of which 71% (1,210) also received gastroprotection. Of the patient episodes who were not prescribed gastroprotection, 46% (223/483) met the criterion of age as a risk factor for gastroprotection. A further 30 episodes met other risk criteria of certain concomitant drugs or prior comorbidity
- Our study provides further evidence on the possible suboptimal management of the use of gastroprotective therapy in hospitalised patients who are receiving DAPT
Conflicts of interest
None declared.
Funding
None.
Study approval
This study was categorised as a service evaluation, not requiring NHS Research Ethics Committee approval. This study was approved locally as a Clinical Effectiveness Project. As data collection occurred within standard clinical care, routinely provided at the study site, patient consent was neither sought nor required. Patient data were used in accordance with local NHS Trust Policy and in line with general data protection regulations.
References
1. Tersalvi G, Biasco L, Cioffi GM et al. Acute coronary syndrome, antiplatelet therapy, and bleeding: a clinical perspective. J Clin Med 2020;9:2064. https://doi.org/10.3390/jcm9072064
2. Valgimigli M, Bueno H, Byrne RA et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018;39:213–60. https://doi.org/10.1093/eurheartj/ehx419
3. Abraham NS, Hlatky MA, Antman EM et al. ACCF/ACG/AHA 2010 expert consensus document on the concomitant use of proton pump inhibitors and thienopyridines: a focused update of the ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use. Circulation 2010;122:2619–33. https://doi.org/10.1161/CIR.0b013e318202f701
4. National Institute for Health and Care Excellence (NICE). Clinical Knowledge Summary. Scenario: Antiplatelet treatment for secondary prevention of cardiovascular disease (CVD). London: NICE, 2020. Available from: https://cks.nice.org.uk/topics/antiplatelet-treatment/management/secondary-prevention-of-cvd/
5. Bhatt DL, Cryer BL, Contant CF et al.; on behalf of the COGENT Investigators. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med 2010;363:1909–17. https://doi.org/10.1056/NEJMoa1007964
6. Vaduganathan M, Cannon CP, Cryer BL et al.; on behalf of the COGENT Investigators. Efficacy and safety of proton-pump inhibitors in high-risk cardiovascular subsets of the COGENT trial. Am J Med 2016;129:1002–05. https://doi.org/10.1016/j.amjmed.2016.03.042
7. Almufleh A, Ramirez FD, So D et al. H2 receptor antagonists versus proton pump inhibitors in patients on dual antiplatelet therapy for coronary artery disease: a systematic review. Cardiology 2018;140:115–23. https://doi.org/10.1159/000489165
8. Royal Cornwall Hospitals NHS Trust. Management of acute chest pain of suspected cardiac origin (unstable angina/NSTEMI) in Cornwall Policy. June 2020.
9. Sehested TSG, Carlson N, Hansen PW et al. Reduced risk of gastrointestinal bleeding associated with proton pump inhibitor therapy in patients treated with dual antiplatelet therapy after myocardial infarction. Eur Heart J 2019;40:1963–70. https://doi.org/10.1093/eurheartj/ehz104
10. Roffi M, Patrono C, Collet JP et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267–315. https://doi.org/10.1093/eurheartj/ehv320
11. Morneau KM, Reaves AB, Martin JB et al. Analysis of gastrointestinal prophylaxis in patients receiving dual antiplatelet therapy with aspirin and clopidogrel. J Manag Care Pharm 2014;20:187–93. https://doi.org/10.18553/jmcp.2014.20.2.187
12. Badar A, Scaife J, Yan AT et al. Provision of gastroprotective medication and bleeding risk following acute coronary syndrome. J Invasive Cardiol 2013;25:397–401. Available from: https://www.hmpgloballearningnetwork.com/site/jic/articles/provision-gastroprotective-medication-and-bleeding-risk-following-acute-coronary-syndrome
13. Nishtala PS, Jamieson HA, Hanger HC et al. Examining the risks of major bleeding events in older people using antithrombotics. Cardiovasc Drugs Ther 2019;33:323–9. https://doi.org/10.1007/s10557-019-06867-z
14. Howard RL, Avery AJ, Slavenburg S et al. Which drugs cause preventable admissions to hospital? A systematic review. Br J Clin Pharmacol 2006;63:136–47. https://doi.org/10.1111/j.1365-2125.2006.02698.x
15. Lghoul-Oulad Saïd F, Hek K, Flinterman LE et al. Prevalence and incidence rate of hospital admissions related to medication between 2008 and 2013 in The Netherlands. Pharmacoepidemiol Drug Saf 2020;29:1659–68. https://doi.org/10.1002/pds.5122
16. Mejía G, Saiz-Rodríguez M, Gómez de Olea B et al. Urgent hospital admissions caused by adverse drug reactions and medication errors – a population-based study in Spain. Front Pharmacol 2020;11:734. https://doi.org/10.3389/fphar.2020.00734
17. NHS England. Annex B – Investment and Impact Fund (IIF): 2021/22 and 2022/23. London: NHS England, 2021. Available from: https://www.england.nhs.uk/wp-content/uploads/2021/08/B0828-iii-annex-b-investment-and-impact-fund-21-22-22-23.pdf
18. NHS Business Services Authority, NHS Digital. Medication safety indicators specification. Newcastle-upon-Tyne: NHSBSA, 2019. Available from: https://www.nhsbsa.nhs.uk/sites/default/files/2019-08/Medication%20Safety%20-%20Indicators%20Specification%20%28Aug19%29.pdf
19. Pirmohamed M, James S, Meakin S et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ 2004;329:15–19. https://doi.org/10.1136/bmj.329.7456.15
20. Guo H, Ye Z, Huang R. Clinical outcomes of concomitant use of proton pump inhibitors and dual antiplatelet therapy: a systematic review and meta-analysis. Front Pharmacol 2021;12:694698. https://doi.org/10.3389/fphar.2021.694698
21. Otero MJ, Moreno-Gómez AM, Santos-Ramos B et al. Developing a list of high-alert medications for patients with chronic diseases. Eur J Intern Med 2014;25:900–08. https://doi.org/10.1016/j.ejim.2014.10.021
22. Otero MJ, Guzmán MDT, Galván-Banqueri M et al. Utility of a trigger tool (TRIGGER-CHRON) to detect adverse events associated with high-alert medications in patients with multimorbidity. Eur J Hosp Pharm 2021;28(suppl 2):s41–s46. https://doi.org/10.1136/ejhpharm-2019-002126
23. Alshakrah MA, Steinke DT, Tully PM et al. Development of the adult complexity tool for pharmaceutical care (ACTPC) in hospital: a modified Delphi study. Res Social Adm Pharm 2021;17:1907–22. https://doi.org/10.1016/j.sapharm.2021.02.009
24. Wallis KA, Elley CR, Moyes S, Kerse N. Safer Prescribing and Care for the Elderly (SPACE): a pilot study in general practice. BJGP Open 2018;2:bjgpopen18X101594. https://doi.org/10.3399/bjgpopen18X101594
25. Peek N, Gude W, Keers RN et al. Evaluation of a pharmacist-led actionable audit and feedback intervention for improving medication safety in UK primary care: an interrupted time series analysis. PLoS Med 2020;17:e1003286. https://doi.org/10.1371/journal.pmed.1003286
26. Shen H, Sestier M, Soltani I et al. Gastroprotection in patients on antithrombotic therapy: a quality improvement study. Can J Cardiol 2021;37:S37–S38. https://doi.org/10.1016/j.cjca.2021.07.080
27. Medicines and Healthcare products Regulatory Agency. Drug safety update. Clopidogrel and proton pump inhibitors: interaction-updated advice. London: MHRA, 2014. Available from: https://www.gov.uk/drug-safety-update/clopidogrel-and-proton-pump-inhibitors-interaction-updated-advice
28. Care Quality Commission. GP myth buster 12: accessing medical records during inspections. London: CQC, 2022. Available from: https://www.cqc.org.uk/guidance-providers/gps/gp-mythbuster-12-accessing-medical-records-during-inspections
29. Makunts T, Cohen IV, Awdishu L et al. Analysis of postmarketing safety data for proton-pump inhibitors reveals increased propensity for renal injury, electrolyte abnormalities, and nephrolithiasis. Sci Rep 2019;9:2282. https://doi.org/10.1038/s41598-019-39335-7
30. Veettil SK, Sadoyu S, Bald EM et al. Association of proton-pump inhibitor use with adverse health outcomes: a systematic umbrella review of meta-analyses of cohort studies and randomised controlled trials. Br J Clin Pharmacol 2022;88:1551–66. https://doi.org/10.1111/bcp.15103
