The evidence supporting early discharge after TAVI

Patients discharged early from hospital generally have a reduced risk of the physical and functional consequences associated with immobilisation and longer hospital stay, including hospital-acquired infections, functional dependency and cognitive decline.13,14 Elderly and frail patients are at risk of hospital-associated complications and are most likely to benefit from a shorter hospital stay.7,14
In the context of TAVI, the feasibility and safety of early discharge are well established.6–10,15 As early as 2015, early discharge was shown to be feasible and safe in approximately 80% of patients.6,7 In the North American 3M TAVR (The Vancouver 3M [Multidisciplinary, Multimodality But Minimalist] Clinical Pathway Facilitates Safe Next-Day Discharge Home at Low-, Medium-, High-Volume Transfemoral Transcatheter Aortic Valve Replacement Centres) study, 89.5% of patients were safely discharged home within 48 hours of a TAVI procedure, and over 80% within 24 hours.8 (https://www.jacc.org/doi/10.1016/j.jcin.2018.12.020) A Belgian study of matched patient outcomes before and after the transition to a fast-track (two to four days length of stay) TAVI pathway found no differences in all-cause mortality, risk of new permanent pacemaker implantation, or rehospitalisation rates at three months.15 In a meta analysis of eight studies involving 1,775 patients undergoing TAVI, Kotronias et al. found that patients were less likely to be re-admitted after early (up to three days) versus standard discharge.10
The most appropriate early discharge target for a fast-track TAVI pathway is yet to be defined. Timing is heavily dependent on local resources, institutional protocols and healthcare systems, all of which differ between countries.16 In this article, we discuss the adoption of a 48-hour target for early discharge to reflect an achievable goal across diverse regions and healthcare systems; we acknowledge that studies have demonstrated the feasibility of next day discharge following TAVI,8,17 and even same-day discharge for highly selected patients to mitigate the competing demands of the COVID-19 pandemic on health resources.18
Achieving safe, early discharge
While the benefits of early discharge after TAVI are known, the processes required to achieve this target are less well established, and many centres struggle to reach a discharge goal within 48 hours of admission. In the European FAST-TAVI (Feasibility and Safety of Early Discharge After Transfemoral Transcatheter Aortic Valve Implantation) trial, logistical reasons contributed to a delay in hospital discharge for over a third of patients.9 (https://eurointervention.pcronline.com/article/optimizing-patient-discharge-management-after-transfemoral-transcatheter-aortic-valve-implantation-the-multicentre-european-fast-tavi-trial) To achieve consistent early discharge, all aspects of care must be scrutinised to identify and address the inefficiencies and barriers within the local systems and protocols.
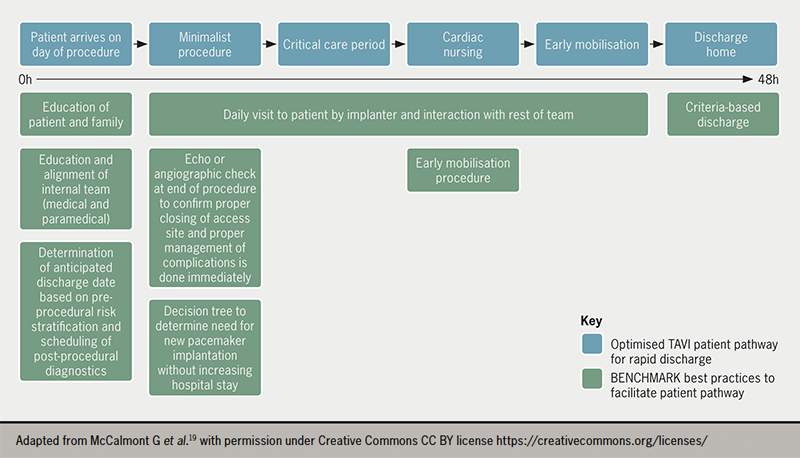
The international, multicentre BENCHMARK registry has recently been developed to assess the implementation of evidence-based quality of care measures to facilitate early discharge following TAVI without impacting patient safety.19 The eight best practices outlined in the BENCHMARK registry19 (see figure 1) include:
- education of the patient/family on early discharge expectations
- education and alignment of the internal team
- determination of an anticipated discharge date at admission
- post-procedural imaging to confirm appropriate management of complications
- early mobilisation four to six hours after the intervention
- early and consistent assessment of the need for a pacemaker
- daily patient visits by the implanter and criteria based discharge.
Role of the TAVI coordinator
Implementing a streamlined TAVI patient pathway requires engagement and buy-in from all major stakeholders, including the TAVI Heart Team (e.g. the interventional cardiologist, cardiac surgeon, anaesthetist, TAVI nurse, procedure room team, cardiac care team, post-procedural nurses), other hospital staff (e.g. cardiac programme administration, care coordinators), and patients and their families. The availability of a designated TAVI coordinator improves the pathway efficiency and increases patient satisfaction.20 The coordinator should be the single point of contact for patients and their relatives from referral to follow-up. The TAVI coordinator is responsible for ensuring timely and appropriate communication, not only within and between the hospital teams but also with stakeholders outside the hospital (e.g. general cardiologist, referring physician); and for systematically assessing patients’ health status and urgency for intervention when waiting for TAVI. This essential role has been endorsed by international guidelines to ensure processes of care, early discharge planning and follow-up to address patient and programme needs.12
Specialised cardiovascular nurses are considered the most appropriate choice for the role of TAVI coordinator based on their clinical and programme development expertise.11,21 In addition to their competencies in the assessment of patients’ evolving clinical presentation and individualised needs, nurses offer leadership in streamlining patient pathways, advocating for resources and monitoring patient access and quality of care.21
According to current guidelines, shared decision-making (SDM) should be at the centre of treatment decisions in AS, with the consideration of patients’ preferences and priorities to achieve an individualised treatment approach and higher patient satisfaction.22 Given the complexity of treatment decisions and organisational constraints, physicians and the TAVI multidisciplinary team may find SDM challenging in the context of severe AS, and its application is not routine in older patients.23,24 In spite of these perceived barriers, SDM minimally extends initial outpatient appointments and leads to higher-quality treatment decisions and patient satisfaction. Therefore, TAVI teams should promote patient participation in SDM to ensure high-quality care according to the principles set out in the treatment guidelines.23
Peri-procedural optimisation
Patients entering a fast-track TAVI pathway should ideally be admitted on the day of the procedure to avoid an unnecessary overnight stay and mitigate the risks of bedrest and in-hospital complications.12 Most patients are suitable for early discharge;8 several preprocedural factors have been identified as predictors of successful TAVI and/or a short hospital length of stay, including good patient mobility,25,26 a pre-existing pacemaker and absence of acute kidney injury before TAVI.6 It is important, however, to identify patients for whom rapid discharge may not be appropriate or feasible. This may include high-risk patients who cannot receive care according to the standardised TAVI clinical pathway for various reasons, including clinical presentation, peri- or post-procedural complications, or lack of social support.6,27 The assessment of frailty using established tools provides additional information to assess individuals’ functional status and appropriate timing for safe discharge. However, baseline frailty scores alone are not a reliable predictor for early or late discharge.
When preparing for early discharge, clear communication with patients, family members and caregivers is key to promote consistent information and encourage patients and their families to prepare a plan for returning home. Pre-operative consultations should focus on explaining the entire treatment pathway to patients, highlighting the benefits of early discharge and beginning the process of discharge planning. A clear target of the expected discharge day based on local practice (e.g. next-day, post-procedure day 2) should be emphasised by every care provider at every time point of interactions with patients and their families. The TAVI coordinator is ideally positioned to ensure consistency and repetition of strategies for supporting patient motivation and participation for early discharge planning.
The adoption of a minimalist TAVI procedure is well established,28 and is well suited to achieve early discharge. Invasive lines should be avoided or minimised, including not using a central venous catheter/arterial monitoring and/or urinary catheter. Local anaesthesia may offer several advantages over general anaesthesia, including a shorter procedure time, decreased requirements for critical care and a shorter length of stay, the avoidance of delirium or functional decline, reduced need for inotropic support, lower 30-day mortality and reduced costs.29–32 Peri-procedure teams should be ready to manage potential peri-procedure complications, such as severe haemodynamic instability or transitioning to general anaesthesia in the event of emergencies.12 Ultrasound-guided vascular access may reduce the risk of vascular access complications during procedures,33 and hence should be favoured to facilitate predictable access site haemostasis and early mobilisation.
Post-procedural optimisation
An effective post-procedure protocol was developed in the 3M TAVR study comprising three parallel activities: patient monitoring, reconditioning and discharge planning (figure 2).34
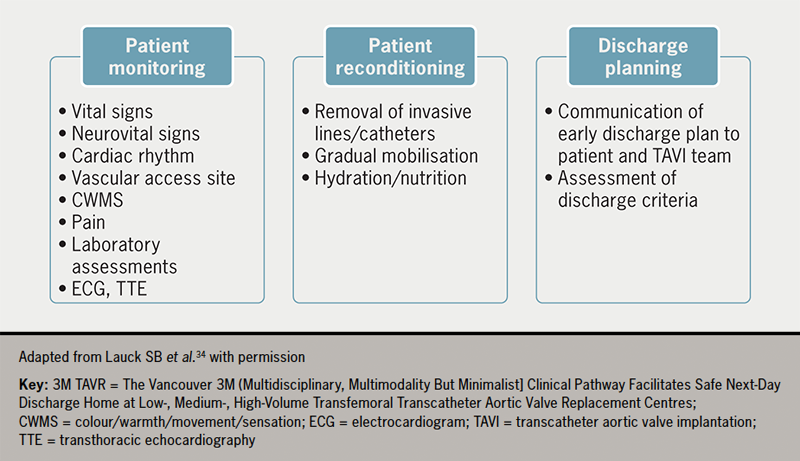
Following the procedure, patients should be assessed regularly for vital signs, neurological status, cardiac rhythm, vascular access haemostasis and pain. Most TAVI patients do not experience significant pain; discomfort and mild pain can be treated with simple analgesic agents. Importantly, sedatives or opioids should not be used in order to avoid delirium. Transthoracic echocardiography (TTE) should be conducted to examine device position, paravalvular leak (PVL) and biventricular function, and laboratory tests for haemoglobin, complete blood counts and renal function should be conducted.34 The standardised scheduling and review of post-procedure TTE can prevent delays to early discharge.
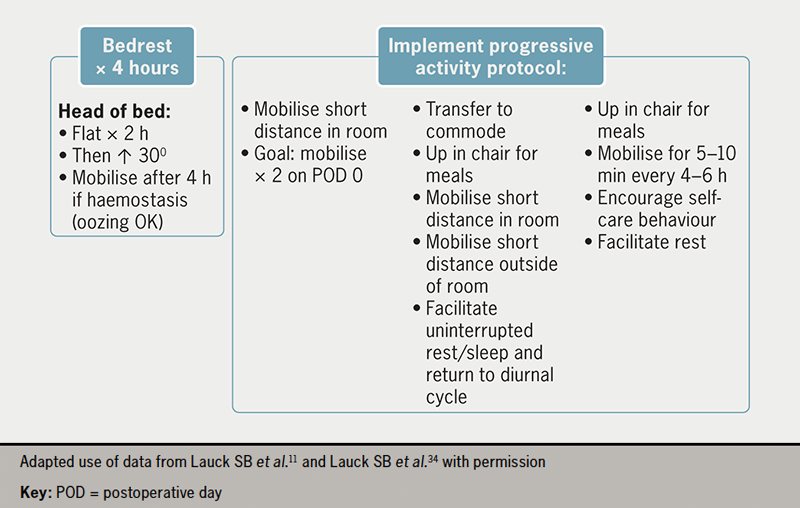
Early mobilisation is a critical component of a fast-track TAVI programme. The post-procedure protocol developed in the 3M TAVR study includes an effective nurse-led protocol for standardised mobilisation four to six hours after TAVI. This evidence-based nursing care standard can be adapted according to individual hospital resources. Before mobilisation, any invasive lines need to be removed at the earliest time point. Progressive mobilisation can then begin, as detailed in figure 3, provided there are no vascular or other complications. A target of walking twice (e.g. to the toilet) on postoperative day 0 facilitates early reconditioning and the avoidance of complications. Mobilisation must be tailored to individual patients and care should be taken to ensure that any aids typically used by the patient, such as a walking stick, walker or spectacles, are in place. Appropriate hydration and encouragement to resume regular meals are important to facilitate a return to baseline health status.34
Prespecified criteria should be defined and met before a patient can be safely discharged home (table 1). The specified list of discharge criteria should be agreed upon within the institution based on local resources and should include the availability of post-discharge support for the patient.
Table 1. Proposed discharge criteria (based on European FAST-TAVI and 3M TAVR studies)9,34
| Discharge criteria |
Multidisciplinary consensus of readiness for discharge and physician’s order considerations may include:
|
| Return to baseline mobilisation |
| Confirmed availability of family member for 24 hours to remain with patient |
| Discharge teaching completed and confirmed follow-up plan |
Successful completion of the streamlined clinical pathway is dependent on good communication, continuity of care, and management of expectations, with nurses playing a crucial role in facilitating post-procedural pathways. Good communication with patients and their families is required to ensure patients are discharged to a safe setting with appropriate care. Communication beyond discharge is also important to ensure the patient continues to be engaged with their rehabilitation. In this respect, the TAVI coordinator may contact the patient by telephone on the day after discharge and at longer follow-up to assess symptoms, recovery and health status, and may assist in ensuring post-procedural blood tests are offered in some places to reduce the risks associated with delayed complications.12 Cardiac rehabilitation services are available in some areas to support patients after discharge through regular communications, home exercise programmes and group classes. These services provide patients with support and advice on lifestyle changes and how to improve risk factors following TAVI.
Educational tools to support a fast-track TAVI pathway
Educational tools, such as information leaflets (e.g. Your TAVI [https://www.rbht.nhs.uk/sites/nhs/files/PILs/TAVI.pdf]) and videos (e.g. ‘Explain my procedure’ https://www.explainmyprocedure.com/procedure/transcatheter-aortic-valve-implantation-tavi-english/ [a multi-language tool]) can help patients to understand and engage with the patient pathway. Educational tools should also be developed to support hospital teams. These may include decision trees, flowcharts and checklists to facilitate passage along the patient pathway, the management of complications, criteria for early discharge and scenarios for individual patient groups. Hospital-led education of referring colleagues is also required so that appropriate patients can gain timely access to a TAVI programme.
Cost benefits of a fast-track TAVI pathway
The study of cost savings and effectiveness of fast-track TAVI highlights opportunities for significant savings related to short hospital admission, avoidance of critical care resources, and mitigation of risk of complications.35 For intermediate-risk patients in the PARTNER II™ (Placement of Aortic Transcatheter Valves II – XT Intermediate) trial, reductions in hospital length-of-stay in patients treated with the latest balloon-expandable TAVI valve led to a $4,000 cost-saving compared with SAVR.35 A fast-track TAVI pathway, focusing on early discharge, would be expected to reduce costs even further. Indeed, a US cost analysis comparing standard TF-TAVI with a minimalist approach that reduced hospital length-of-stay by two days found an almost $10,000 cost-saving with the fast-track pathway.36 Economic analyses based on the PARTNER 3™ (Safety and Effectiveness of the SAPIEN 3™ Transcatheter Heart Valve in Low Risk Patients with Aortic Stenosis) trial have also shown cost-savings with TAVI versus SAVR in low-risk patients.37,38 Together, these studies suggest that a TAVI pathway focusing on the mitigation of in-hospital risks and early discharge would reap benefits not only from a patient’s perspective but also for resource use.
Conclusion
Care goals for elderly, fragile patients requiring treatment for AS are consistent across institutions. However, resources differ substantially between regions, so local practices must be adapted accordingly. There have been several drivers for a systematic, standardised approach to AS treatment. First, the number of patients requiring treatment is increasing dramatically, driven by an ageing population, a greater appreciation of the burden of asymptomatic severe AS, and expansion of TAVI into lower-risk groups. Second, the COVID-19 pandemic has emphasised the need for a more streamlined pathway to shorten hospital stays and reduce hospital-based complications. Upfront investment in a well informed, structured and standardised approach can lead to earlier discharge, better patient outcomes and fewer complications. With careful attendance to documented best practices and full support from the entire TAVI team, centres can move towards an optimised patient pathway that achieves consistently safe, early discharge for patients. This approach is effective from a patient care perspective and can lead to substantial cost savings and resource efficiencies.
Key messages
- A successful transcatheter aortic valve implantation (TAVI) programme aims to resolve aortic stenosis with minimal short- and long-term complications, enabling patients to be discharged efficiently and safely
- Early reconditioning and safe discharge of eligible patients after TAVI reduces the risk of hospital-associated complications without increasing the risk of mortality, new permanent pacemaker implantation or re-hospitalisation
- The adoption of evidence-based best practices and the full support from the multidisciplinary TAVI team enable centres to consistently achieve earlier discharge, better patient outcomes and fewer complications, alongside substantial cost savings and resource efficiencies to improve access to care
Conflicts of interest
ED, SL and DF are consultants for Edwards Lifesciences. JR has received consultancy fees from Edwards Lifesciences.
Funding
Professor Eric Durand has received a grant from the French Government managed by the National Research Agency (ANR) under the program ‘Investissements d’avenir’ (reference ANR-16-RHUS-0003) and is also supported by a grant from the GCS G4 as part of the FHU-CARNAVAL, labeled by AVIESAN.
Eric Durand
Professor of Cardiology
Normandie University, UNIROUEN, U1096, CHU Rouen, Department of Cardiology, FHU CARNAVAL, F-76000 Rouen, France
Sandra Lauck
Clinician Scientist , St Paul’s Hospital, and Associate Professor, St Paul’s Hospital Professorship in Cardiovascular Nursing
University of British Columbia, Vancouver, Canada
Derk Frank
Professor of Cardiology and Director
Internal Medicine III at UKSH, Arnold-Heller-Straße 3, 24105 Kiel, Germany, and German Centre of Cardiovascular Research (DZHK), partner site Hamburg, Lübeck, Kiel, Germany
John Rawlins
Consultant Interventional Cardiologist
Coronary Research Group, University Hospital Southampton NHS Foundation Trust, Southampton, SO16 6YD, UK
Correspondence to:
SLauck@providencehealth.bc.ca
Articles in this supplement
Introduction: overcoming barriers to treating severe aortic stenosis
The past, present and future of aortic stenosis treatment
A standardised network to improve the detection and referral of patients with aortic stenosis
Further information: cost-benefit analyses of TAVI
Some information on the cost-benefit analyses of TAVI can be found in the following links:
Gilard M, Eltchaninoff H, Iung B et al. Transcatheter aortic valve implantation procedure compared with surgery in patients with severe aortic stenosis at low risk or surgical mortality in France. Value Health 2022;25:605–13. https://doi.org/10.1016/j.jval.2021.10.003
https://www.sciencedirect.com/science/article/pii/S1098301521017861
Hospital Healthcare Europe. Economic implications and cost effectiveness of TAVI. https://hospitalhealthcare.com/edwards/economic-implications-and-cost-effectiveness-of-tavi/ (last accessed 10th November 2022)
Lorenzoni V, Barbieri G, Saia F et al. The cost-effectiveness of transcatheter aortic valve implantation: exploring the Italian National Health System perspective and different patient risk groups. Eur J Health Econ 2021;22:1349–63. https://doi.org/10.1007/s10198-021-01314-z
https://link.springer.com/article/10.1007/s10198-021-01314-z
Mennini FS, Meucci F, Pesarini G et al. Cost-effectiveness of transcatheter aortic valve implantation versus surgical aortic valve replacement in low surgical risk aortic stenosis patients. Int J Cardiol 2022;357:26–32. https://doi.org/10.1016/j.ijcard.2022.03.034
https://www.sciencedirect.com/science/article/pii/S016752732200393X
Rodríguez JMV, Bermúdez EP, Zamorano JL et al. Cost-effectiveness of SAPIEN 3™ transcatheter aortic valve implantation in low surgical mortality risk patients in Spain. REC Interv Cardiol 2022; published online 19th October 2022. https://doi.org/10.24875/RECICE.M22000340
https://recintervcardiol.org/en/original-article/cost-effectiveness-of-sapien-3-transcatheter-aortic-valve-implantation-in-low-surgical-mortality-risk-patients-in-spain
References
1. Roth GA, Mensah GA, Johnson CO et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 study. J Am Coll Cardiol 2020;76:2982–3021. https://doi.org/10.1016/j.jacc.2020.11.010
2. d’Arcy JL, Coffey S, Loudon MA et al. Large-scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people: The OxVALVE Population Cohort Study. Eur Heart J 2016;37:3515–22. https://doi.org/10.1093/eurheartj/ehw229
3. Roule V, Rebouh I, Lemaitre A et al. Impact of wait times on late postprocedural mortality after successful transcatheter aortic valve replacement. Sci Rep 2022;12:5967. https://doi.org/10.1038/s41598-022-09995-z
4. Joseph J, Kotronias RA, Estrin-Serlui T et al. Safety and operational efficiency of restructuring and redeploying a transcatheter aortic valve replacement service during the COVID-19 pandemic: The Oxford experience. Cardiovasc Revasc Med 2021;31:26–31. https://doi.org/10.1016/j.carrev.2020.12.002
5. Tan J, Teoh K, Ivanova J et al. The impact of the COVID-19 pandemic on transcatheter aortic valve implantation (TAVI) services in the United Kingdom: A tertiary centre experience. Heart 2021;107:A13–A14. https://doi.org/10.1136/heartjnl-2021-BCS.17
6. Durand E, Eltchaninoff H, Canville A et al. Feasibility and safety of early discharge after transfemoral transcatheter aortic valve implantation with the Edwards SAPIEN-XT™ prosthesis. Am J Cardiol 2015;115:1116–22. https://doi.org/10.1016/j.amjcard.2015.01.546
7. Serletis-Bizios A, Durand E, Cellier G et al. A prospective analysis of early discharge after transfemoral transcatheter aortic valve implantation. Am J Cardiol 2016;118:866–72. https://doi.org/10.1016/j.amjcard.2016.06.035
8. Wood DA, Lauck SB, Cairns JA et al. The Vancouver 3M (Multidisciplinary, Multimodality, But Minimalist) clinical pathway facilitates safe next-day discharge home at low-, medium-, and high-volume transfemoral transcatheter aortic valve replacement centers: The 3M TAVR study. JACC Cardiovasc Interv 2019;12:459–69. https://doi.org/10.1016/j.jcin.2018.12.020
9. Barbanti M, van Mourik MS, Spence MS et al. Optimising patient discharge management after transfemoral transcatheter aortic valve implantation: The multicentre European FAST-TAVI trial. EuroIntervention 2019;15:147–54. https://doi.org/10.4244/EIJ-D-18-01197
10. Kotronias RA, Teitelbaum M, Webb JG et al. Early versus standard discharge after transcatheter aortic valve replacement: A systematic review and meta-analysis. JACC Cardiovasc Interv 2018;11:1759–71. https://doi.org/10.1016/j.jcin.2018.04.042
11. Lauck SB, Wood DA, Baumbusch J et al. Vancouver Transcatheter Aortic Valve Replacement clinical pathway: Minimalist approach, standardized care, and discharge criteria to reduce length of stay. Circ Cardiovasc Qual Outcomes 2016;9:312–21. https://doi.org/10.1161/CIRCOUTCOMES.115.002541
12. Lauck S, Forman J, Borregaard B et al. Facilitating transcatheter aortic valve implantation in the era of COVID-19: Recommendations for programmes. Eur J Cardiovasc Nurs 2020;19:537–44. https://doi.org/10.1177/1474515120934057
13. Tirado-Conte G, Freitas-Ferraz AB, Nombela-Franco L et al. Incidence, causes, and impact of in-hospital infections after transcatheter aortic valve implantation. Am J Cardiol 2016;118:403–9. https://doi.org/10.1016/j.amjcard.2016.05.012
14. Calero-García MJ, Ortega AR, Navarro E et al. Relationship between hospitalization and functional and cognitive impairment in hospitalized older adults patients. Aging Ment Health 2017;21:1164–70. https://doi.org/10.1080/13607863.2016.1220917
15. Baekke PS, Jørgensen TH, Søndergaard L. Impact of early hospital discharge on clinical outcomes after transcatheter aortic valve implantation. Catheter Cardiovasc Interv 2021;98:E282–E90. https://doi.org/10.1002/ccd.29403
16. Lutz M, Messika-Zeitoun D, Rudolph TK et al. Differences in the presentation and management of patients with severe aortic stenosis in different European centres. Open Heart 2020;7(2)e001345. https://doi.org/10.1136/openhrt-2020-001345
17. Gupta R, Mahajan S, Mehta A et al. Next-day discharge vs early discharge after transcatheter aortic valve replacement: Systematic review and meta-analysis. Curr Probl Cardiol 2021;47:100998. https://doi.org/10.1016/j.cpcardiol.2021.100998
18. Pop AM, Barker M, Hickman L et al. Same day discharge during the COVID-19 pandemic in highly selected transcatheter aortic valve replacement patients. Struct Heart 2021;5:596–604. https://doi.org/10.1080/24748706.2021.1988780
19. McCalmont G, Durand E, Lauck S et al. Setting a benchmark for resource utilization and quality of care in patients undergoing transcatheter aortic valve implantation in Europe – Rationale and design of the international BENCHMARK registry. Clin Cardiol 2021;44:1344–53. https://doi.org/10.1002/clc.23711
20. Bohmann K, Burgdorf C, Zeus T et al. The COORDINATE Pilot Study: Impact of a transcatheter aortic valve coordinator program on hospital and patient outcomes. J Clin Med 2022;11:1205. https://doi.org/10.3390/jcm11051205
21. Lauck SB, McGladrey J, Lawlor C et al. Nursing leadership of the transcatheter aortic valve implantation Heart Team: Supporting innovation, excellence, and sustainability. Healthc Manage Forum 2016;29:126–30. https://doi.org/10.1177/0840470416632004
22. Vahanian A, Beyersdorf F, Praz F et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 2022;43:561–632. https://doi.org/10.1093/eurheartj/ehab395
23. van Beek-Peeters J, van Noort EHM, Faes MC et al. Shared decision making in older patients with symptomatic severe aortic stenosis: A systematic review. Heart 2020;106:647–55. https://doi.org/10.1136/heartjnl-2019-316055
24. van Beek-Peeters J, van der Meer JBL, Faes MC et al. Professionals’ views on shared decision-making in severe aortic stenosis. Heart 2022;108:558–64. https://doi.org/10.1136/heartjnl-2021-320194
25. Cockburn J, Singh MS, Rafi NH et al. Poor mobility predicts adverse outcome better than other frailty indices in patients undergoing transcatheter aortic valve implantation. Catheter Cardiovasc Interv 2015;86:1271–7. https://doi.org/10.1002/ccd.25991
26. Amin R, Arunothayaraj S, Kirtchuk D et al. Mobility aids predict mortality after transcatheter aortic valve implantation. Catheter Cardiovasc Interv 2022;99:e31–e37. https://doi.org/10.1002/ccd.29981
27. Durand E, Avinée G, Gillibert A et al. Analysis of length of stay after transfemoral transcatheter aortic valve replacement: Results from the FRANCE TAVI registry. Clin Res Cardiol 2021;110:40–9. https://doi.org/10.1007/s00392-020-01647-4
28. Sawan MA, Calhoun AE, Grubb KJ et al. Update on Minimalist TAVR Care Pathways: Approaches to Care in 2022. Curr Cardiol Rep 2022;24:1179–87. https://doi.org/10.1007/s11886-022-01737-x
29. Villablanca PA, Mohananey D, Nikolic K et al. Comparison of local versus general anesthesia in patients undergoing transcatheter aortic valve replacement: A meta-analysis. Catheter Cardiovasc Interv 2018;91:330–42. https://doi.org/10.1002/ccd.27207
30. Lauck S, Wood D, Sathananthan J et al. Anesthesia for TAVR patients: Should we focus on goals of care? Structural Heart 2020;4:310–11. https://doi.org/10.1080/24748706.2020.1774950
31. Herrmann HC, Cohen DJ, Hahn RT et al. Utilization, costs, and outcomes of conscious sedation versus general anesthesia for transcatheter aortic valve replacement. Circ Cardiovasc Interv 2021;14:e010310. https://doi.org/10.1161/CIRCINTERVENTIONS.120.010310
32. Husser O, Fujita B, Hengstenberg C et al. Conscious sedation versus general anesthesia in transcatheter aortic valve replacement: The German Aortic Valve Registry. JACC Cardiovasc Interv 2018;11:567–78. https://doi.org/10.1016/j.jcin.2017.12.019
33. Kotronias RA, Scarsini R, De Maria GL et al. Ultrasound guided vascular access site management and left ventricular pacing are associated with improved outcomes in contemporary transcatheter aortic valve replacement: Insights from the OxTAVI registry. Catheter Cardiovasc Interv 2020;96:432–9. https://doi.org/10.1002/ccd.28578
34. Lauck SB, Sathananthan J, Park J et al. Post-procedure protocol to facilitate next-day discharge: Results of the multidisciplinary, multimodality but minimalist TAVR study. Catheter Cardiovasc Interv 2020;96:450–8. https://doi.org/10.1002/ccd.28617
35. Baron SJ, Wang K, House JA et al. Cost-effectiveness of transcatheter versus surgical aortic valve replacement in patients with severe aortic stenosis at intermediate risk. Circulation 2019;139:877–88. https://doi.org/10.1161/CIRCULATIONAHA.118.035236
36. Babaliaros V, Devireddy C, Lerakis S et al. Comparison of transfemoral transcatheter aortic valve replacement performed in the catheterization laboratory (minimalist approach) versus hybrid operating room (standard approach): Outcomes and cost analysis. JACC Cardiovasc Interv 2014;7:898–904. https://doi.org/10.1016/j.jcin.2014.04.005
37. Santé HAd. SAPIEN 3™ modèle 9600 TFX bioprothese valvulaire aortique avec systeme de mise en place Edwards COMMANDER™. 2021. Available at: https://www.has-sante.fr/jcms/p_3244168/en/sapien-3-modele-9600-tfx-bioprothese-valvulaire-aortique-avec-systeme-de-mise-en-place-edwards-commander [last accessed 4 May 2022].
38. Health NIoP. Transcatheter aortic valve implantation (TAVI) versus surgical aortic valve replacement (SAVR) for patients with severe aortic stenosis and low surgical risk and across surgical risk groups: A health technology assessment. 2021. Available at: https://www.fhi.no/en/publ/2021/TAVI-vs-SAVR-for-patients-with-severe-aortic-stenosis-and-low-surgical-risk-and-across-surgical-risk-groups/ [last accessed 31 May 2022].

