While statins are the gold standard for lipid-lowering therapies, newer therapies, such as PCSK9 inhibitors, have also demonstrated low-density lipoprotein cholesterol (LDL-C) reduction, but with a similar or better safety profile. Conflicting guidance has contributed to a low uptake. More up-to-date, evidence-led guidance supports greater use of newer therapies, particularly in combination with statins, to reduce LDL-C to levels shown to be effective in trials. The aim of this study was to determine how such guidance can be implemented more effectively in the UK.
Using a modified Delphi approach, a panel of healthcare professionals with an interest in the management of dyslipidaemia developed 27 statements across four key themes. These were used to form an online survey that was distributed to healthcare professionals working in cardiovascular care across the UK. Stopping criteria included 100 responses received, a seven-month window for response (September 2021 to March 2022), and 90% of statements passing the predefined consensus threshold of 75%.
A total of 109 responses were analysed with 23 statements achieving consensus (four statements <75%). Variance was observed across respondent role, and by UK region. From the high degree of consensus, seven recommendations were established as to how evidence-based guidance can be delivered, including a call for personalised therapy strategies and simplification of LDL-C goals, which should be achieved within as short a time as possible.
Introduction
Statins are the gold-standard lipid-lowering therapy based on their efficacy in reducing serum low-density lipoprotein cholesterol (LDL-C) and general tolerability.1 While statins have an extensive body of evidence that have shown them to reduce the risk of cardiovascular (CV) events,2,3 there are concerns around side effects. An increase in cases of treatment-induced comorbidities, such as new-onset diabetes mellitus (NODM) has been observed.4-6 When combined with patient and media concerns, this has led to a reported 50% drop-out rate within 12 months.1
In response, other LDL-C lowering medications have been developed. Proprotein convertase subtilisin-kexin type 9 inhibitors (PCSK9i), such as alirocumab and evolocumab, reduce LDL-C by approximately 60%, reaching maximal reduction within four weeks at a similar safety profile to other interventions.7 PCSK9i have also been shown to reduce inflammation and progression of atherosclerotic disease.8
Despite inclusion in the Lipid Management – Rapid Uptake Product programme,7 the number of prescriptions issued for PCSK9i remains far below projected levels (figure 1).
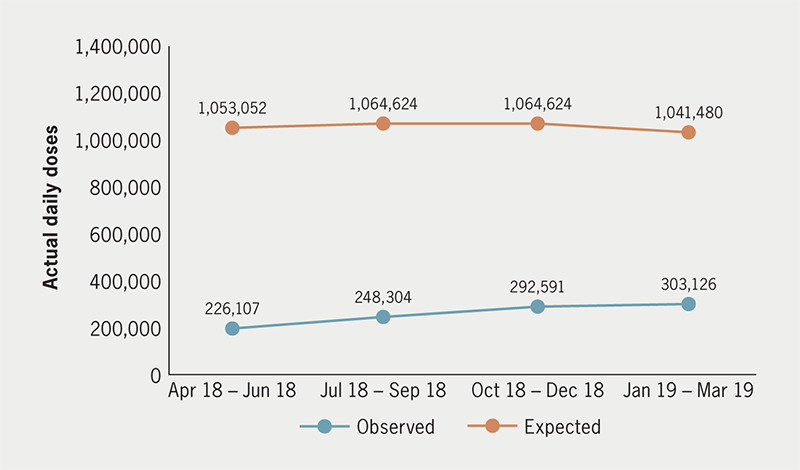
The National Institute for Health and Care Excellence (NICE) recommends starting PCSK9i for patients with LDL-C concentrations persistently above 4.0/3.5 mmol/L, despite maximally tolerated lipid-lowering therapy (including statins, high-intensity statins, and combination therapy with ezetimibe).10 This is problematic as NICE guidelines for cardiovascular disease (CVD) specify a 40% reduction in serum non-high density lipoprotein cholesterol (non-HDL-C, defined as total cholesterol minus HDL-C) as a treatment goal rather than specifying goals based on particular LDL-C levels.11 In contrast, European Society of Cardiology (ESC) guidelines focus on LDL-C as a marker for CVD risk.12
These issues are further compounded through familial hypercholesterolaemia (FH). While a relatively common genetic disorder with a prevalence of between one in 250 and one in 500,13,14 with an estimated 120,000–260,000 individuals affected in the UK,13,14 85% are unaware they have the condition.13 As a result, untreated men are at a 50% risk of a coronary event before 50 years of age, with women at a 30% risk before 60 years.15
Though evidence suggests PCSK9i are effective in getting FH patients to target,16 the current NICE guidelines state that PCSK9i can only be prescribed in those individuals with uncontrolled LDL-C or do not achieve a 50% reduction from baseline.17 NICE further recommends starting FH patients without CVD and at high/very high risk of CVD only if LDL-C concentration is persistently above 5.0/3.5 mmol/L, respectively.10
Confusion regarding lipid parameters to measure (LDL-C vs. non-HDL-C) may result in hesitancy among National Health Service (NHS) prescribers to escalate treatment (e.g. to include PCSK9i and bempedoic acid, along with novel treatments such as inclisiran), resulting in UK patients receiving suboptimal treatment.
Given these points, the objective of this project was to determine the strength of opinion held by UK healthcare professionals (HCPs) involved in lipid care as to how evidence-based guidance can be implemented, and what role newer therapies can have in delivering these guidelines.
Method
A panel of experts in the management of CVD from across the UK convened in July 2021 to discuss current challenges in LDL-C reduction and how additional treatment options could be utilised to achieve these goals. Using a modified Delphi methodology guided by an independent facilitator, the panellists identified four main topics of focus:
- Why do we need to address current practice in lipid management?
- Defining a treatment target
- Best practice principles
- Considerations (targets) for other populations.
These topics were each discussed further, from which 27 statements were developed for wider testing using an online questionnaire using Microsoft Forms. The questionnaire was distributed through a convenience sampling18 method to HCPs working within CVD care across the UK. No incentive was provided to any responder. Stopping criteria were defined as a seven-month time period to collect responses (September 2021 to March 2022), more than 90% of statements exceeding the threshold established for consensus, and a minimum target of 100 responses within the pre-defined time period.
The threshold for consensus agreement was defined as 75%. This was further defined as ‘high’ at ≥75% and ‘very high’ at ≥90%. Respondents were offered a four-point Likert scale (‘strongly disagree’, ‘tend to disagree’, ‘tend to agree’, and ‘strongly agree’) to indicate their corresponding level of agreement with each statement. The questionnaire also captured some demographic data for further analyses, including country of work, role, and time in role.
Completed, anonymised surveys were collected and analysed by an independent facilitator to produce an arithmetic agreement score for each statement. This information was then reviewed by the panellists and recommendations made accordingly.
As this study only sought the anonymous opinions of HCPs and no patient-specific data were captured, ethical approval was not required.
Results
A total of 109 responses from across the UK were received between September 2021 and March 2022 (figure 2).
From this first round, 14/27 statements attained very high agreement (≥90%), 9/27 attained high agreement (<90% and ≥75%), and 4/27 statements did not reach the threshold for consensus (<75%).
Given the high level of agreement displayed to the statements, and that the stopping criteria had been met, it was decided not to undertake a second round of testing.
The defined consensus statements and corresponding levels of agreement from 109 responses are shown in table 1.
Table 1. Defined consensus statements and corresponding levels of agreement from 109 responses
| No. | Statement | Score % |
|---|---|---|
| Topic 1: Why do we need to address current practice in lipid management? | ||
| 1 | The lifetime burden of LDL-C has significant healthcare consequences | 100 |
| 2 | Current risk calculations are underestimating the lifetime burden of LDL-C and its consequences | 94 |
| 3 | There is conflicting guidance on appropriate lipid targets with NICE versus ESC | 97 |
| 4 | Conflicting guidance on appropriate lipid targets in the UK leads to suboptimal health outcomes | 90 |
| 5 | More intensive lipid management in the UK will lead to improved patient outcomes | 94 |
| 6 | An evidence-based, population-based approach to target setting should be the goal in the UK | 89 |
| 7 | The European Society of Cardiology (ESC) 2019 targets should be adopted in the UK | 88 |
| Topic 2: Defining a treatment target | ||
| 8 | The use of non-HDL-C targets in the UK leads to confusion in the application of clinical guidelines | 82 |
| 9 | Non-HDL-C should no longer be used in routine clinical practice in the UK | 69 |
| 10 | An LDL-C target should be the standard of measurement in future UK guidance | 80 |
| 11 | It is important to get all patients with high LDL-C to the treatment target as quickly as possible | 87 |
| 12 | LDL-C targets should apply equally irrespective of the lipid-lowering therapy being used | 93 |
| 13 | For patients with high CV risk, the absolute treatment target for LDL-C should be 1.8 mmol/L and a ≥50% reduction from baseline LDL-C | 93 |
| 14 | For patients with very-high CV risk, the absolute treatment target for LDL-C should be 1.4 mmol/L and a ≥50% reduction from baseline LDL-C | 89 |
| 15 | Secondary prevention ASCVD patients should be initiated on a combination of a high-dose statin and ezetimibe following their initial ASCVD event | 72 |
| 16 | After treatments to reduce LDL-C have been initiated, treatment should be reviewed (and optimised) within 3 months | 96 |
| 17 | The addition of PCSK9 inhibitors to lipid management improves patient outcomes | 95 |
| 18 | PCSK9 inhibitors should be prescribed in primary care | 73 |
| 19 | In my practice there are barriers to prescribing PCSK9 inhibitors | 74 |
| 20 | The current NICE guidance for the use of PCSK9 inhibitors is too restrictive | 84 |
| Topic 3: Best practice principles | ||
| 21 | Combination therapy for lipid management should be recommended, as is the case for hypertension and diabetes | 95 |
| 22 | If patients fit the NICE criteria for a PCSK9 inhibitor, they should be initiated on this option rather than bempedoic acid (± ezetimibe) | 87 |
| Topic 4: Considerations (targets) for other populations | ||
| 23 | The nocebo effect should be considered before patients are considered statin intolerant (the nocebo effect is the opposite of the placebo effect. It describes a situation where a negative outcome occurs due to a belief that the intervention will cause harm) | 94 |
| 24 | Other lipid-lowering therapies should always be considered in patients for whom statins cannot be used | 99 |
| 25 | Other lipid-lowering treatment options should always be considered in patients that experience side effects | 96 |
| 26 | Patients with known CVD, type 2 diabetes, very high levels of individual risk factors and chronic kidney disease (CKD) are at high or very-high total cardiovascular risk and need active management of all risk factors | 100 |
| 27 | Patients with known CVD, type 1 diabetes with microalbuminuria are at high or very-high total cardiovascular risk and need active management of all risk factors | 100 |
| Key: ASCVD = atheroasclerotic cardiovascular disease; CV = cardiovascular; CVD = cardiovascular disease; ESC = European Society of Cardiology; LDL-C = low-density lipoprotein cholesterol; NICE = National Institute for Health and Care Excellence; non-HDL-C = non-high-density lipoprotein cholesterol; PCSK9 = proprotein convertase subtilisin-kexin type 9 | ||
Discussion
Research-led guidelines with a focus to personalised treatment goals should become the gold standard within the UK
All respondents recognised that the concept of lifetime burden of LDL-C in predisposing an individual to atheromatous CVD is currently underappreciated (S1, 100%). This has potential implications for patients, as delaying treatment through a misconception of lipids being a ‘slow-burning’ condition will lead to adverse consequences for the individual if their lipids are left unoptimised. This is compounded by the lack of provision to support personalised LDL-C goals within NICE guidelines,11 contrary to recent research findings.19
Given the levels of agreement in statements 3 and 7 (97% and 88%), it is clear that HCPs recognise that the ESC guidelines are more appropriate to manage individual patient needs. This is in part due to the stratification of targets according to risk category alongside the personalisation of LDL-C goals for each patient.12,20 As these guidelines are also frequently updated in line with current research, the authors suggest that ESC guidelines should be adopted within the UK.
Focus on LDL-C rather than non-HDL-C
The use of non-HDL-C goals within the UK exacerbate confusion around patient lipid goals. A subanalysis of agreement against respondent role demonstrates the differences in opinion between lipid specialists and other roles, with the other specialist disciplines demonstrating a marked lower level of agreement to statement 9. Education for all HCPs should, therefore, be improved to reduce variation in understanding of lipid goals. However, current NICE guidance uses both non-HDL-C and LDL-C goals,11,21 the agreement for statement 10 demonstrates that HCPs recognise that LDL-C should be the standard unit of measurement in future guidance, as currently supported by the ESC.12
The urgency of treatment is as important as the treatment used
The lifetime burden of LDL-C is recognised as a key issue (S1, 100%). What is evident is that the respondents are aware of the need to lower LDL-C as fast as possible (S11, 87%). This result corresponds to the accepted viewpoint in the field that ‘lower is better’ as it has been shown that a 1.0 mmol/L reduction in LDL-C corresponds to a 22% reduction in relative risk.16,22
Statin monotherapy is no longer the only treatment for lipid management
Since the introduction of statins, there are now five main available therapies for use, with different combinations and approaches available. It is important that these options are employed in an evidence-based approach.
While respondents agreed that there should be one LDL-C target regardless of the therapy used (S12, 93%), care should be taken to employ the most efficacious combinations. Recent data show that a combination of statins and bempedoic acid is only 16% more effective in reducing LDL-C compared with other methods.23 Within high-risk groups, statin monotherapy is often less effective, warranting combination therapy use, a position supported by recent research,18,20,24 to the point of starting those most at risk immediately on statin/non-statin combination therapy.20
Furthermore, respondents agree it is vital to ensure that treatments are reviewed and optimised within three months to ensure an individual meets their LDL-C goals (S16, 96%).
Combination therapies are key to getting patients to achieve goals
Respondents indicated very strong agreement (S21, 95%) that combination therapies20 should be used to achieve goals, as is the case for hypertension and glucose lowering in type 2 diabetes mellitus.
As a first step, widening the usage of PCSK9i would be the logical approach. The utility of PCSK9i is recognised by HCPs as shown by the degree of support for statements 17, 20, and 22 (95%, 84%, and 87%, respectively). However, as NICE guidance around their use is recognised to be too restrictive (S20, 84%), the authors suggest that addressing the barriers inhibiting their use would increase uptake. Potential barriers may include:
- a lack of a multi-disciplinary approach
- confusion around goals for implementation and requirements to measure LDL-C
- a lack of experience and knowledge of PCSK9i
- a need to improve patient knowledge and engagement of this class of treatment.
The authors also welcome the population health agreement between the NHS in England and the manufacturer of inclisiran to provide a further tool for the treatment of CVD, but suggest similar options for the other patented treatments so more combination therapies can be delivered.
Recommendations
Based on the levels of agreement, the authors offer the following set of recommendations:
- Tools for managing the lifetime risk of LDL-C should be better implemented in clinical practice.
- Lipid-lowering therapies should always be personalised, optimised within as short a time period as possible, and all patients should be brought to their goals as quickly as possible.
- Goals for lipid-lowering therapies should be the same across all therapeutic classes and reflect the evidence-based LDL-C goals set by the ESC guidelines (1.4–3.0 mmol/L for very-high-risk to low-risk patients).
- LDL-C should be the standard unit of measurement in future UK guidance.
- Use of combination therapy should become normal, and treatments made widely accessible.
- Awareness around the advantages of PCSK9i monoclonal antibodies, inclisiran and bempedoic acid should be raised for both appropriate patients and for HCPs.
- There needs to be recognition of intensive treatment options for high-risk populations.
In summary, two key drivers to improve the lipid care pathways for patients emerge. The first is that NICE guidelines should follow the standards set by the evidence-led ESC. The second is that there needs to be investment in education and support around the early implementation of newer therapies, such as PCSK9i, to ensure that patients goals are optimised swiftly. Achieving these key drivers for patients throughout the UK will improve patient quality of life (QoL) and CVD outcomes.
The results represent the opinions of a representative sample of practising professionals within the field, in response to questions generated by a panel of experts, and provide a reflective grounding towards the current state of CVD care as practised in primary and secondary care settings.
As a consensus study, the data represent a qualitative sample, and the evidence tier may reduce the impact of the findings. As convenience sampling methods were used, the results may have been affected by motivation bias, however, this was mitigated by the seven-month time frame within the study design. As most results originated from England, this may have weighted the results.
Key messages
- Despite up-to-date, evidence-led guidance supporting greater use of newer therapies in combination with statins to reduce low-density lipoprotein cholesterol (LDL-C), uptake in the UK is low
- Using a modified Delphi-based methodology, a panel of 109 UK lipid specialists were sampled to determine how evidence-based guidance can be better implemented in the UK through a 27 statement Delphi consensus survey
- A set of seven recommendations were derived from the results to inform future guideline development, including a call for personalised therapy strategies and simplification of LDL-C goals, which should be achieved within as short a time period as possible
Conflicts of interest
DLC has received research funding or advisory board/speaker fees from Amgen, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Daiichii Sankyo, Novartis, Novo Nordisk, Pfizer, Sanofi-Aventis. SCB has received personal fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Novo Nordisk, and Sanofi-Aventis. SCB is a shareholder in Glycosmedia. KF has received funding from Amgen, Novartis and Daiichi-Sankyo. AZ has received funding from Amgen, Sanofi, Amarin, Novartis, AstraZeneca and Pfizer. NEC: none declared.
Funding
The study was initiated and funded by Amgen Ltd. All authors received funding from Amgen Ltd. while attending meetings when undertaking this study. Amgen Ltd. commissioned Triducive Partners Limited to facilitate the project and analyse the responses to the consensus statements in line with the Delphi methodology.
Acknowledgements
The authors wish to thank Tim Warren and Thomas Scoble from Triducive Partners Limited for their support in analysing the results, writing the manuscript, and reviewing the final draft.
Study approval
As this study only sought the anonymous opinions of HCPs and no patient-specific data were captured, ethical approval was not required.
References
1. Kasichayanula S, Grover A, Emery M et al. Clinical pharmacokinetics and pharmacodynamics of evolocumab, a PCSK9 inhibitor. Clin Pharmacokinet 2018;57:769–79. https://doi.org/10.1007/s40262-017-0620-7
2. Cholesterol Treatment Trialists’ Collaboration. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet 2015;385:1397–405. https://doi.org/10.1016/S0140-6736(14)61368-4
3. Koskinas KC, Siontis GCM, Piccolo R et al. Effect of statins and non-statin LDL-lowering medications on cardiovascular outcomes in secondary prevention: a meta-analysis of randomized trials. Eur Heart J 2018;39:1172–80. https://doi.org/10.1093/eurheartj/ehx566
4. Adhyaru BB, Jacobson TA. Safety and efficacy of statin therapy. Nat Rev Cardiol 2018;15:757–69. https://doi.org/10.1038/s41569-018-0098-5
5. Chrysant SG. New onset diabetes mellitus induced by statins: current evidence. Postgrad Med 2017;129:430–5. https://doi.org/10.1080/00325481.2017.1292107
6. Keni R, Sekhar A, Gourishetti K et al. Role of statins in new-onset diabetes mellitus: the underlying cause, mechanisms involved, and strategies to combat. Curr Drug Targets 2021;22:1121–8. https://doi.org/10.2174/1389450122666210120125945
7. NHS England. NHS Accelerated Access Collaborative. Lipid management – rapid uptake product. Available at: https://www.england.nhs.uk/aac/what-we-do/what-innovations-do-we-support/rapid-uptake-products/lipid-management/ [accessed 18 May 2022].
8. Ding Z., Pothineni NV, Goel A et al. PCSK9 and inflammation: role of shear stress, pro-inflammatory cytokines, and LOX-1. Cardiovasc Res 2020;116:908–15. https://doi.org/10.1093/cvr/cvz313
9. NHS Digital. NICE technology appraisals in the NHS in England (innovation scorecard) to March 2019. Estimates report. Available at: https://digital.nhs.uk/data-and-information/publications/statistical/nice-technology-appraisals-in-the-nhs-in-england-innovation-scorecard/to-march-2019/2.-estimates-report#primary-hypercholesterolaemia-and-mixed-dyslipidaemia [accessed 18 May 2022].
10. National Institute for Health and Care Excellence. Evolocumab for treating primary hypercholesterolaemia and mixed dyslipidaemia. TA394. London: NICE, 2016. Available from: https://www.nice.org.uk/guidance/ta394
11. National Institute for Health and Care Excellence. Cardiovascular disease: risk assessment and reduction, including lipid modification. CG181. London: NICE, 2016. Available from: https://www.nice.org.uk/guidance/cg181/
12. Mach F, Baigent C, Catapano A et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2019;41:111–88. https://doi.org/10.1093/eurheartj/ehz455
13. Public Health England. Familial hypercholesterolaemia: implementing a systems approach to detection and management. London: Public Health England, 2018. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/731873/familial_hypercholesterolaemia_implementation_guide.pdf
14. NHS England. Briefing: familial hypercholesterolaemia in England. London: NHS England, 2013. Available from: https://www.england.nhs.uk/wp-content/uploads/2013/11/fh_eEngland-briefing11_2013.pdf
15. Youngblom E, Pariani M, Knowles JW. Familial hypercholesterolemia. In: Adam MP, Ardinger HH, Pagon RA et al. (eds). GeneReviews. Seattle (WA): University of Washington, Seattle, 1993–2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK174884/
16. Rosenson RS, Hegele RA, Fazio S, Cannon CP. The evolving future of PCSK9 inhibitors. J Am Coll Cardiol 2018;72:314–29. https://doi.org/10.1016/j.jacc.2018.04.054
17. National Institute for Health and Care Excellence. Familial hypercholesterolaemia: identification and management. CG71. London: NICE, 2019. Available from: https://www.nice.org.uk/guidance/cg71
18. Battaglia M. Convenience sampling. In: Lavrakas PJ (ed). Encyclopedia of Survey Research Methods. Sage Publications, Inc., 2008; pp. 149. https://doi.org/10.4135/9781412963947.n105
19. De Backer G, Jankowski P, Kotseva K et al. Management of dyslipidaemia in patients with coronary heart disease: results from the ESC-EORP EUROASPIRE V survey in 27 countries. Atherosclerosis 2019;285:135–46. https://doi.org/10.1016/j.atherosclerosis.2019.03.014
20. Ray K, Reeskamp L, Laufs U et al. Combination lipid-lowering therapy as first-line strategy in very high-risk patients. Eur Heart J 2021;43:830–3. https://doi.org/10.1093/eurheartj/ehab718
21. Khatib R, Neely D; on behalf of the Accelarated Access Collaborative Clinical Subgroup. Summary of national guidance for lipid management for primary and secondary prevention of CVD. London: NHS England, November 2022. Available from: https://www.england.nhs.uk/aac/publication/summary-of-national-guidance-for-lipid-management/
22. Packard C, Chapman MJ, Sibartie M, Laufs U, Masana L. Intensive low-density lipoprotein cholesterol lowering in cardiovascular disease prevention: opportunities and challenges. Heart 2021;107:1369–75. https://doi.org/10.1136/heartjnl-2020-318760
23. Ray K, Bays H, Catapano A et al. Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N Engl J Med 2019;380:1022–32. https://doi.org/10.1056/NEJMoa1803917
24. Ray K, Molemans B, Schoonen W et al. EU-wide cross-sectional observational study of lipid-modifying therapy use in secondary and primary care: the DA VINCI study. Eur J Prev Cardiol 2020;28:1279–89. https://doi.org/10.1093/eurjpc/zwaa047
