Atrial fibrillation (AF) is a common arrhythmia associated with poor outcomes. N-3 fatty acids have been shown to provide significant cardiovascular risk reduction, but they may exacerbate the risk of AF. The pathway by which N-3 fatty acids may be arrhythmogenic is unknown. One possible mechanism involves cardiac chamber morphology alteration. The purpose of this study was to investigate the effect of icosapent ethyl (IPE) on left atrial (LA) size and left ventricular (LV) mass.
This study used coronary computed tomographic angiography images gathered from the Effect of Icosapent Ethyl on Progression of Coronary Atherosclerosis (EVAPORATE) trial. EVAPORATE was a randomised, double-blind, placebo-controlled study finding a significant reduction in coronary atherosclerosis progression in patients with residually elevated triglycerides despite statin therapy on 4 g IPE daily versus 4 g placebo daily. Computed tomography images were used to measure LA size and LV mass at 0 and 18 months.
Of 80 enrolled patients, 68 were included in the final analysis. Baseline demographics and risk factors were similar between IPE and placebo cohorts. LA anterior-posterior diameter measured on axial (p=0.51) and sagittal (p=0.52) orientations were not different over time. Also, there was no difference between groups in the change in LA volume (p=0.84). Change in LV mass was similar between groups (p=0.13).
In conclusion, this study did not detect differences in LA size or LV mass over 18 months between patients on 4 g daily IPE versus placebo.
Introduction
Atrial fibrillation (AF) is a common arrhythmia with significant associated morbidity, mortality, and healthcare costs.1 N-3 fatty acids may influence the risk of AF, but previous studies show conflicting evidence on whether N-3 fatty acids are pro- or anti-arrhythmogenic. Given the significant cardiovascular disease risk reduction associated with N-3 fatty acids, there has been interest in delineating their risk profile.
In 2004, Mozaffarian et al. noted that increased dietary fish intake was associated with lower incidence of AF.2 Two separate studies suggested that higher levels of circulating long-chain N-3 fatty acid and docosahexaenoic acid (DHA) levels were associated with lower risk of incident AF.3,4 In contrast, the Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia (REDUCE-IT) trial, Long-Term Outcomes Study to Assess Statin Residual Risk With Epanova in High Cardiovascular Risk Patients With Hypertriglyceridemia (STRENGTH) trial, and the Effects of N-3 Fatty Acid Supplements in Elderly Patients After Myocardial Infarction (OMEMI) trial showed an increase in rates of AF in the N-3 group.5–7 Of note, REDUCE-IT used icosapent ethyl (IPE), which consists almost entirely of eicosapentaenoic acid (EPA), while STRENGTH and OMEMI used a combination of EPA and DHA. A systematic review of randomised-controlled trials concluded that increased N-3 fatty acid intake reduced the risk of AF, but noted there was only marginal statistical significance, substantial heterogeneity, and very low quality evidence.8 Additionally, this same review noted that there seemed to be a protective effect of N-3 fatty acids in shorter trials, but harm in longer trials.
Given these nebulous findings, it is important to continue examinations. One outstanding question is the mechanism by which N-3 fatty acids may alter the incidence of AF. There are many risk factors for AF, including both left atrial enlargement (LAE) and left ventricular hypertrophy (LVH).9–11 Previously, a population study showed no difference in cardiac chamber size associated with serum levels of EPA and DHA.12 Then, Heydari et al. (2016) showed that N-3 fatty acid supplementation started in patients presenting with acute myocardial infarction led to a reduction in left ventricular remodelling.13 A randomised-controlled trial on the effects of N-3 fatty acid therapy on cardiac chamber morphology in an outpatient setting has not been previously published.
The purpose of this study was to investigate the impact of IPE on LA size and LV mass using coronary computed tomographic angiography (coronary CTA) images from the Effect of Icosapent Ethyl on Progression of Coronary Atherosclerosis in Patients with Elevated Triglycerides on Statin Therapy trial (EVAPORATE).14
Materials and method
The study design, rationale, patient selection, and coronary CTA protocol for EVAPORATE have been published previously.15 Briefly, the objective of EVAPORATE was to study the effects of 4 g of IPE per day as an adjunct to diet and statin therapy, in patients with elevated fasting triglyceride levels, on coronary CTA plaque volumes.
Study end points
The purpose of this study was to investigate the impact of IPE (4 g/day) on LA size (as assessed by LA diameter and LA volume) and LV mass using coronary CTA images from the EVAPORATE trial. The primary end point was the change in LA diameter, LA volume, and LV mass.
Study population
A total of 80 patients were enrolled in this randomised, double-blind, placebo-controlled trial. Inclusion criteria comprised age between 30 and 85 years, known coronary artery disease, elevated fasting triglyceride levels (135–499 mg/dL), and low-density lipoprotein (LDL) levels between ≥40 and ≤115 mg/dL. All patients were to be on stable statin therapy, with or without ezetimibe, diet, and exercise for at least four weeks before entering the study. All patients were instructed to maintain a low cholesterol diet and to continue current statin therapy.
Study design
EVAPORATE was a multi-centre, randomised, double-blind, placebo-controlled trial that evaluated the effect of IPE 4 g/day on coronary plaque progression determined by coronary CTA compared with pharmaceutical grade mineral oil placebo. Patients were randomised 1:1 to IPE or placebo to evaluate progression rates of plaque volume on coronary CTA. Participants underwent a coronary CTA scan at 0 and 18 months. This study was approved by the Institutional Review Board at each site and was conducted in accordance with the principles of Good Clinical Practice and the trial conformed to the principles outlined in the Declaration of Helsinki. All patients provided written informed consent prior to randomisation.
Chamber measurement protocol
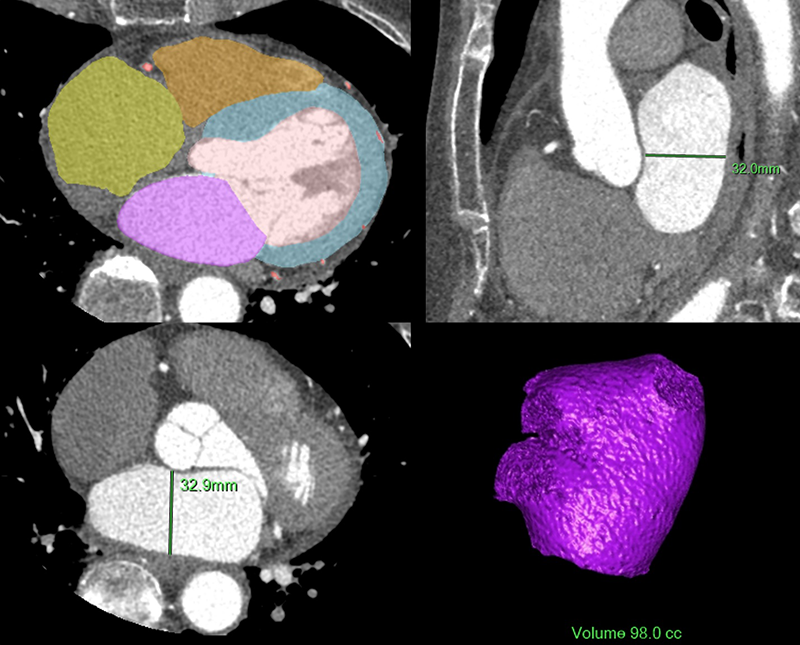
Quantitative data analyses were performed using automated methods on a workstation and software (Philips Intellispace Portal, Philips Healthcare, Cleveland, OH) that used a Hounsfield unit-based endocardial border detection technique and method that was previously validated.16 Images were reconstructed with a 1.25 mm slice thickness. The mid-diastolic phases were chosen for measurements of LA volume, LA diameter, and LV mass (figure 1). The LA appendage and pulmonary veins were not included in the LA volume measurement. LA volume and LV mass were simultaneously calculated automatically. LA diameter was manually measured at the maximum anterior-posterior diameter of the midline in its middle 50% on axial and sagittal orientations, similar to a validated method (figure 1).17
Placebo composition and icosapent ethyl
The total daily dose of placebo was 4 g soft gelatin capsules, to be taken as two capsules twice daily with meals. Active treatment consisted of 4 g IPE, which is a highly purified, stable, ethyl ester of EPA, per day.
Statistical analysis
Chamber size measures are presented as mean ± standard deviation. ANOVA was used to examine the changes in measures between the IPE versus placebo group. A two-sided p value of 0.05 was considered statistically significant. Multi-variate linear regression models adjusted for cardiovascular risk factors were used to evaluate the IPE effect on change in cardiac chamber measures. Outcome variables were log transformed if appropriate. All statistical calculations were performed using SAS (Version 9.4, SAS Inc., Cary, NC).
Results
A total of 80 eligible subjects were enrolled with 68 subjects completing the 18-month visit and having an interpretable coronary CTA at baseline and the 18-month visit. The baseline demographics, risk factors for AF, and laboratory results stratified by arm (IPE group n=31 and placebo group n=37) of the study participants were initially presented in EVAPORATE, and are displayed here in a slightly modified format (table 1).14 Baseline demographics, risk factors, and laboratory results were not significantly different between the IPE and placebo groups.14
Table 1. Baseline characteristics of the EVAPORATE cohort (adapted from Budoff et al. 202014). Comparison of baseline characteristics of the icosapent ethyl (IPE) and placebo groups. There was no difference in baseline demographics or risk factors for atrial fibrillation (AF) between groups
| Characteristic | Total N=68 |
IPE N=31 |
Placebo N=37 |
p value |
|---|---|---|---|---|
| Mean age ± SD, years | 57.4 ± 8.7 | 56.5 ± 8.9 | 58.3 ± 8.6 | 0.394a |
| Male gender, n (%) | 37 (54.4) | 17 (54.8) | 20 (54.1) | 0.948b |
| Mean BMI ± SD, kg/m2 | 33.7 ± 6.7 | 34.1 ± 6.5 | 33.3 ± 6.9 | 0.632a |
| Mean time between visit 1 and 5, months | 17.8 ± 3.8 | 17.2 ± 4.0 | 18.2 ± 3.6 | 0.275a |
| Diabetes mellitus, n (%) | 47 (69.1) | 22 (71.0) | 25 (67.6) | 0.763b |
| Statin use, n (%) | 68 (100) | 31 (100) | 37 (100) | – |
| Hypertension, n (%) | 52 (76.5) | 24 (77.4) | 28 (75.7) | 0.866b |
| Past smoking, n (%) | 29 (42.6) | 13 (41.9) | 16 (43.2) | 0.986b |
| a Independent t-test; b Chi-square test Key: BMI = body mass index; SD = standard deviation |
||||
Baseline inflammatory markers for treatment and placebo groups, including fibrinogen (467.8 ± 105.4 mg/dL vs. 474.8 ± 118.8 mg/dL), lipoprotein-associated phospholipase A2 (122.7 ± 33.0 ng/mL vs. 123.7 ± 45.5 ng/mL), myeloperoxidase (379.1 ± 158.2 µg/L vs. 389.6 ± 152.4 µg/L), and high-sensitivity C-reactive protein (4.2 ± 4.2 mg/L vs. 4.5 ± 4.2 mg/L) were similar between groups. All inflammatory markers, except high-sensitivity C-reactive protein trended down over time in both groups. High-sensitivity C-reactive protein was stable in the treatment group (+0.0 ± 3.6 mg/L) and in the placebo group (+1.1 ± 5.9 mg/L). There was no difference in change in any of the inflammatory markers between groups over time.
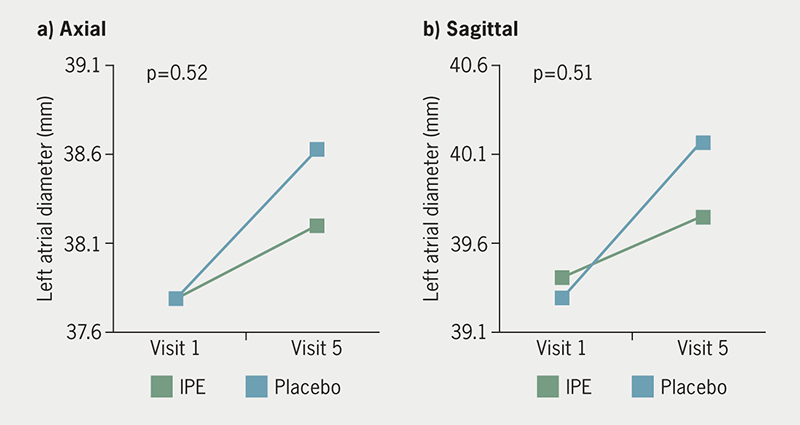
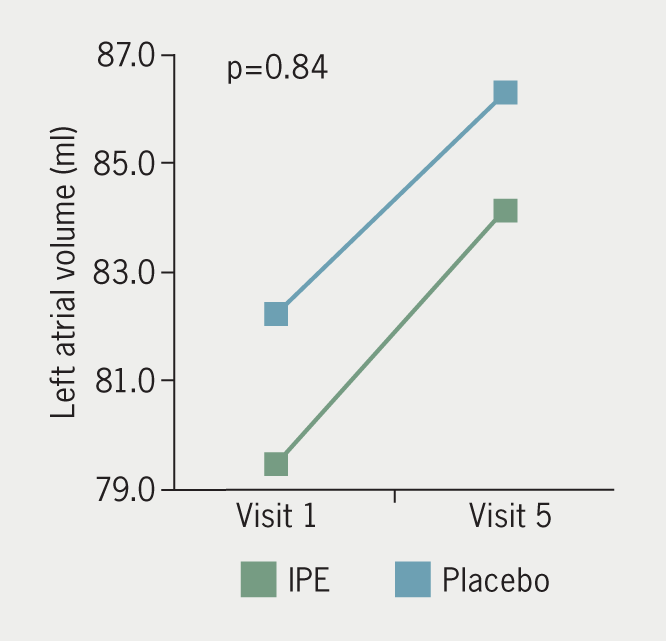
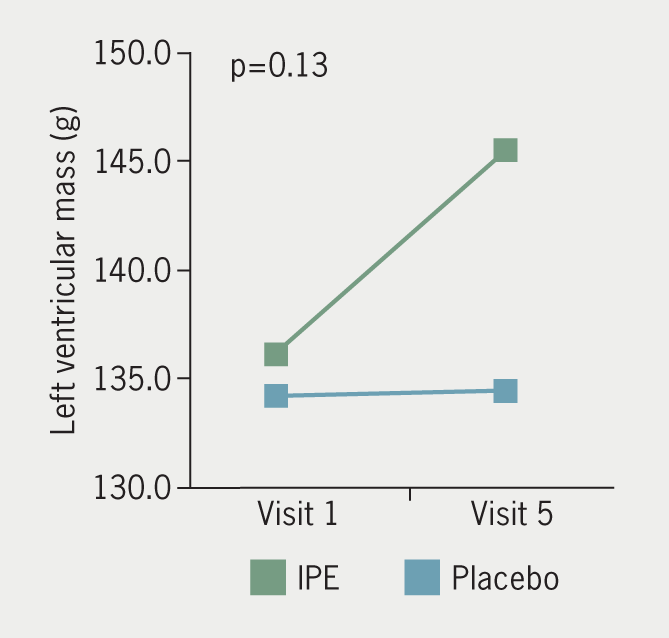
The IPE group had a mean difference of +0.4 ± 2.3 mm and +0.3 ± 3.4 mm in axial and sagittal measurements of LA anterior-posterior diameter over 18 months, respectively. The placebo group had a mean difference of +0.3 ± 3.4 mm and +0.9 ± 3.4 mm in axial and sagittal measurements of LA anterior-posterior diameter, respectively, over the same time period. There was no difference in LA diameter by axial (p=0.51) or sagittal (p=0.52) measurement between groups (figure 2). Mean LA volume change over 18 months was +4.7 ± 11.3 ml in the IPE group and +4.1 ± 11.8 ml in the placebo group, with no between-group difference for LA volume (p=0.84) (figure 3). Mean LV mass mean difference over time was +8.9 ± 26.3 g in the IPE group, and +0.2 ± 8.9 g in the placebo group (figure 4). There was no difference in the change in LV mass over time between groups (p=0.13). In adjusted models, changes in cardiac chamber measures did not demonstrate an IPE effect (p>0.05).
Discussion
N-3 supplementation, whether EPA alone or EPA with DHA, has shown both cardiovascular disease risk benefit and increased risk of AF. Historical data pertaining to the influence of IPE on the risk of AF is unclear. Given the significant cardiovascular disease benefit, it is important to characterise the potential risks of this therapy. IPE did not significantly change LA volume, LA diameter, or LV mass over 18 months as compared with placebo in this trial.
One commonly cited risk factor associated with AF is LA size, assessed by both LA volume and LA diameter.9–11,18–21 LAE, which negatively impacts LA function, is a maladaptive response to a variety of stressors. Such maladaptive remodelling promotes electrical changes in the atria, which may make the LA more prone to re-entry tachycardias.22 Our study did not detect any between-group differences for LA diameter and LA volume. Similarly, a prior population study did not show any echocardiographic evidence of changes to LA size associated with increased serum levels of EPA, though we have extended these findings with a randomised design and using CT imaging.12
LVH is another risk factor that is associated with AF.11,18,23 Several aetiologies for the increased arrhythmogenicity in LVH have been proposed. Electrophysiologic studies have considered the impact of prolongation, or non-uniform propagation, of the action potential throughout the myocardium, as well as slowing and fractionation of ventricular conduction.24,25 Ischaemia, fibrosis, neuroendocrine pathways, and ventricular wall stress have also been identified.24,25 The data presented here show no change in LV mass with IPE therapy as compared with placebo. Other literature on N-3 fatty acids and LVH are sparse and conflicting.
One study showed no significant associations between serum EPA levels and LV mass, but did show a positive correlation between DHA and LV mass in women.26 However, other trials have suggested that higher DHA and EPA serum levels or dietary intake were significantly associated with reduced LV mass.27,28 N-3 fatty acids have shown cardiovascular disease benefit, and LV mass is a marker of cardiovascular disease (CVD) outcomes. Therefore, one may have expected an inverse relationship between N-3 fatty acids and LV mass.29,30 There are several reasons why our study may have different results from previous studies, including trial design, population size, and omega-3 formulation.
Other investigations on LV mass and N-3 fatty acids include population and dietary intervention trials, compared with the present randomised pharmacologic trial. Next, the various inclusion criteria of these studies were different from EVAPORATE; they did not include having known coronary artery disease or taking statin medication. Also, the serum levels of EPA may have been different between these other studies and EVAPORATE. Although EVAPORATE did not trend serum N-3 fatty acid levels, these were recorded at baseline and at one year in REDUCE-IT, in a similar population with the same dosage and drug, as 26.1 µg/ml at baseline and 144 µg/ml at one year.5 Only one of the aforementioned studies on LVH published absolute baseline serum EPA levels as 70.9 µg/ml and 63.2 µg/ml in the two cohorts, respectively.27 It is unlikely that these other studies reached similar levels of serum EPA as EVAPORATE or REDUCE-IT. Lastly, EVAPORATE used IPE, which is almost exclusively derived from EPA, while the other studies on N-3 fatty acids and LV mass assessed both DHA and EPA.
It is important to delineate the exact omega-3 formulation assessed in each study, because different formulations have shown different clinical outcomes. For example, STRENGTH, which was similarly designed to REDUCE-IT but used a different omega-3 formulation containing both EPA and DHA, was a negative trial. Interestingly, there does not seem to be much difference in the risk of AF so far between EPA alone or EPA in combination with DHA, since REDUCE-IT, STRENGTH, and OMEMI all used different N-3 fatty acid therapies, and all showed increased risk of AF. Interestingly, REDUCE-IT showed a significant reduction in stroke in the treatment group while STRENGTH and OMEMI did not.
Two variables in study design that do seem to influence AF outcomes are duration of follow-up and sample size. This is consistent with the formerly mentioned meta-analysis, which had found evidence that N-3 fatty acids were more likely to be pro-arrhythmic in longer trials.8 EVAPORATE (median follow-up of 1.5 years) did not show an increase in incidence of AF, REDUCE-IT, STRENGTH, and OMEMI had longer median follow-up (5 years, 3 years, 2 years, respectively). The two longer and larger trials, REDUCE-IT (n=8,179) and STRENGTH (n=12,633), both showed an increased risk of AF in the treatment group, although the absolute risk increase was small for both REDUCE-IT (1.4%) and STRENGTH (0.9%).5,7 Meanwhile, there was a trend towards increased risk of AF in OMEMI (n=1,014), but this did not achieve statistical significance.6
Given cardiac chamber morphologies remained stable between groups, alternative risk factors and pathways by which N-3 fatty acids influence AF risk must be scrutinised. Inflammation, cardiac fibrosis, and oxidative stress are commonly cited, closely linked risk factors for AF, which together represent the effects of a variety of clinical conditions, such as obesity, diabetes, age, hypertension, and alcohol use.31-34 These pathways seem less likely to be exacerbated by N-3 fatty acids. Indeed, N-3 fatty acids have been shown to be anti-inflammatory across multiple pathways.35-37 As an example, REDUCE-IT and the Effect of Omega-3 Acid Ethyl Esters on Left Ventricular Remodeling After Acute Myocardial Infarction (OMEGA-REMODEL) showed a reduction in serum biomarkers of inflammation.5,13 Interestingly, our study showed no difference in inflammatory markers between groups, which is likely a function of a smaller sample size and different clinical population as compared with these other studies. OMEGA-REMODEL assessed patients suffering an acute myocardial infarction, while EVAPORATE was an outpatient study. The significant pro-inflammatory state in the setting of acute myocardial infarction may have contributed to a larger effect size of the anti-inflammatory N-3 fatty acids. N-3 fatty acids are also thought to have both anti-fibrotic and anti-oxidant properties based on cell, animal, and human studies.13,38-43 However, data on the end effect of N-3 fatty acids on myocardial tissue characteristics and AF substrate formation is very limited.
One of the limitations of this study is the relatively small sample size. Further, the duration of EVAPORATE was originally designed to detect changes in plaque progression, for which 18 months is reasonable. In this instance, 18 months may have been insufficient to detect changes in cardiac chamber sizes. Moreover, LA functional analysis and myocardial tissue assessments were not done in this investigation. Alterations in both measures may predict and/or precede AF, even in the absence of morphologic changes.
Conclusion
This is the first study to evaluate the longitudinal effect of IPE on LA size and LV mass in an outpatient population. There was no effect of IPE on LA size or LV mass compared with placebo. Thus, changes in LA and LV gross anatomy are unlikely to be the culprit for the observed increase in AF incidence in studying omega-3 supplements.
Key messages
- N-3 fatty acids influence the risk of atrial fibrillation
- Icosapent ethyl is a therapeutic N-3 fatty acid that consists of nearly entirely eicosapentaenoic acid and has been found to both reduce cardiovascular risk and increase the rate of atrial fibrillation, via an unknown mechanism, in patients with residually elevated triglycerides despite maximally tolerated statin therapy
- The present study evaluated left atrial and left ventricular morphologic changes over time in patients on icosapent ethyl compared with patients on placebo over an 18-month period, utilising computed tomography images, and found no difference in left atrial diameter, left atrial volume, or left ventricular mass
- Alternative mechanisms by which icosapent ethyl causes an increased risk of atrial fibrillation should be further investigated to more completely understand the risk profile of this drug
Conflicts of interest
MGR: Consultant for HeartFlow. DLB: Advisory boards for Boehringer Ingelheim, Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Janssen, Level Ex, Medscape Cardiology, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, and Stasys; Board of Directors for Boston VA Research Institute, DRS.LINQ (stock options), Society of Cardiovascular Patient Care, and TobeSoft; Inaugural Chair, American Heart Association Quality Oversight Committee; Data Monitoring Committees: Acesion Pharma, Assistance Publique-Hôpitaux de Paris, Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Boston Scientific (Chair, PEITHO trial), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Novartis, Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Chair, ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol-Myers Squibb clopidogrel litigation), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Cowen and Company, Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Piper Sandler, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Afimmune, Aker Biomarine, Amarin, Amgen, AstraZeneca, Bayer, Beren, Boehringer Ingelheim, Bristol-Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CSL Behring, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Lilly, Medtronic, Moderna, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Owkin, Pfizer, PhaseBio, PLx Pharma, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, 89Bio; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Abbott, Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Philips, Svelte; Trustee: American College of Cardiology; Unfunded research: FlowCo, Merck, Takeda. MJB: Amarin grant support and speakers bureau, General electric grant support. SSK, SL, AK, SSM: None declared.
Funding
EVAPORATE, from which this study utilised data and images, was funded by Amarin Pharma, Inc. (Bridgewater NJ, USA). As an investigator-initiated study (MJB), the company had no input in analysis, end point adjudication, or study performance or measures. Otherwise, this research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Study approval
This study was approved by the Institutional Review Board at each site and was conducted in accordance with the principles of Good Clinical Practice and the trial conformed to the principles outlined in the Declaration of Helsinki. All patients provided written informed consent prior to randomisation.
References
1. Wolf PA, Mitchell JB, Baker CS, Kannel WB, D’Agostino RB. Impact of atrial fibrillation on mortality, stroke, and medical costs. Arch Intern Med 1998;158:229–34. https://doi.org/10.1001/archinte.158.3.229
2. Mozaffarian D, Psaty BM, Rimm EB et al. Fish intake and risk of incident atrial fibrillation. Circulation 2004;110:368–73. https://doi.org/10.1161/01.CIR.0000138154.00779.A5
3. Wu JHY, Lemaitre RN, King IB et al. Association of plasma phospholipid long-chain ω-3 fatty acids with incident atrial fibrillation in older adults: the cardiovascular health study. Circulation 2012;125:1084–93. https://doi.org/10.1161/CIRCULATIONAHA.111.062653
4. Virtanen JK, Mursu J, Voutilainen S, Tuomainen TP. Serum long-chain n-3 polyunsaturated fatty acids and risk of hospital diagnosis of atrial fibrillation in men. Circulation 2009;120:2315–21. https://doi.org/10.1161/CIRCULATIONAHA.109.852657
5. Bhatt DL, Steg PG, Miller M et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019;380:11–22. https://doi.org/10.1056/NEJMoa1812792
6. Kalstad AA, Myhre PL, Laake K et al. Effects of n-3 fatty acid supplements in elderly patients after myocardial infarction. Circulation 2021;143:528–39. https://doi.org/10.1161/CIRCULATIONAHA.120.052209
7. Nicholls SJ, Lincoff AM, Garcia M et al. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA 2020;324:2268–80. https://doi.org/10.1001/jama.2020.22258
8. Abdelhamid AS, Martin N, Bridges C et al. Polyunsaturated fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev 2018;11:CD012345. https://doi.org/10.1002/14651858.CD012345.pub3
9. Henry WL, Morganroth J, Pearlman AS et al. Relation between echocardiographically determined left atrial size and atrial fibrillation. Circulation 1976;53:273–9. https://doi.org/10.1161/01.CIR.53.2.273
10. Keren G, Etzion T, Sherez J et al. Atrial fibrillation and atrial enlargement in patients with mitral stenosis. Am Heart J 1987;114:1146–55. https://doi.org/10.1016/0002-8703(87)90190-6
11. Vaziri SM, Larson MG, Benjamin EJ, Levy D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham heart study. Circulation 1994;89:724–30. https://doi.org/10.1161/01.CIR.89.2.724
12. Reinders I, van Ballegooijen AJ, Visser M et al. Associations of serum n-3 and n-6 polyunsaturated fatty acids with echocardiographic measures among older adults: the Hoorn study. Eur J Clin Nutr 2013;67:1277–83. https://doi.org/10.1038/ejcn.2013.167
13. Heydari B, Abdullah S, Pottala JV et al. Effect of omega-3 acid ethyl esters on left ventricular remodeling after acute myocardial infarction. Circulation 2016;134:378–91. https://doi.org/10.1161/CIRCULATIONAHA.115.019949
14. Budoff MJ, Bhatt DL, Kinninger A et al. Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides on statin therapy: final results of the EVAPORATE trial. Eur Heart J 2020;41:3925–32. https://doi.org/10.1093/eurheartj/ehaa652
15. Budoff M, Brent Muhlestein J, Le VT, May HT, Roy S, Nelson JR. Effect of Vascepa (icosapent ethyl) on progression of coronary atherosclerosis in patients with elevated triglycerides (200–499 mg/dL) on statin therapy: rationale and design of the EVAPORATE study. Clin Cardiol 2018;41:13–19. https://doi.org/10.1002/clc.22856
16. Mao SS, Li D, Vembar M et al. Model-based automatic segmentation algorithm accurately assesses the whole cardiac volumetric parameters in patients with cardiac CT angiography: a validation study for evaluating the accuracy of the workstation software and establishing the reference values. Acad Radiol 2014;21:639–47. https://doi.org/10.1016/j.acra.2014.01.010
17. Lick AN, Danrad R, Smith DL, Lammi MR. Left atrium measurements via computed tomography pulmonary angiogram as a predictor of diastolic dysfunction. J Comput Assist Tomogr 2017;41:792–7. https://doi.org/10.1097/RCT.0000000000000597
18. Shenasa M, Shenasa H, El-Sherif N. Left ventricular hypertrophy and arrhythmogenesis. Card Electrophysiol Clin 2015;7:207–20. https://doi.org/10.1016/j.ccep.2015.03.017
19. McManus DD, Yin X, Gladstone R et al. Alcohol consumption, left atrial diameter, and atrial fibrillation. J Am Heart Assoc 2016;5:e004060. https://doi.org/10.1161/JAHA.116.004060
20. Abhayaratna WP, Seward JB, Appleton CP et al. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol 2006;47:2357–63. https://doi.org/10.1016/j.jacc.2006.02.048
21. Wang TJ, Parise H, Levy D et al. Obesity and the risk of new-onset atrial fibrillation. JAMA 2004;292:2471–7. https://doi.org/10.1001/jama.292.20.2471
22. Thomas L, Abhayaratna WP. Left atrial reverse remodeling: mechanisms, evaluation, and clinical significance. JACC Cardiovasc Imaging 2017;10:65–77. https://doi.org/10.1016/j.jcmg.2016.11.003
23. Seko Y, Kato T, Haruna T et al. Association between atrial fibrillation, atrial enlargement, and left ventricular geometric remodeling. Sci Rep 2018;8:6366. https://doi.org/10.1038/s41598-018-24875-1
24. Chatterjee S, Bavishi C, Sardar P et al. Meta-analysis of left ventricular hypertrophy and sustained arrhythmias. Am J Cardiol 2014;114:1049–52. https://doi.org/10.1016/j.amjcard.2014.07.015
25. Wolk R. Arrhythmogenic mechanisms in left ventricular hypertrophy. EP Eur 2000;2:216–23. https://doi.org/10.1053/eupc.2000.0110
26. Anderson JS, Nettleton JA, Hundley WG et al. Associations of plasma phospholipid omega-6 and omega-3 polyunsaturated fatty acid levels and MRI measures of cardiovascular structure and function: the Multiethnic Study of Atherosclerosis. J Nutr Metab 2011;2011:e315134. https://doi.org/10.1155/2011/315134
27. Yano Y, Hoshide S, Tamaki N et al. Regional differences in hypertensive cardiovascular remodeling between fishing and farming communities in Japan. Am J Hypertens 2011;24:437–43. https://doi.org/10.1038/ajh.2010.263
28. Haufe S, Utz W, Engeli S et al. Left ventricular mass and function with reduced-fat or reduced-carbohydrate hypocaloric diets in overweight and obese subjects. Hypertens Dallas Tex 1979;59:70–5. https://doi.org/10.1161/HYPERTENSIONAHA.111.178616
29. Quiñones MA, Greenberg BH, Kopelen HA et al. Echocardiographic predictors of clinical outcome in patients with left ventricular dysfunction enrolled in the SOLVD registry and trials: significance of left ventricular hypertrophy. J Am Coll Cardiol 2000;35:1237–44. https://doi.org/10.1016/S0735-1097(00)00511-8
30. de Simone G, Izzo R, Aurigemma GP et al. Cardiovascular risk in relation to a new classification of hypertensive left ventricular geometric abnormalities. J Hypertens 2015;33:745–54. https://doi.org/10.1097/HJH.0000000000000477
31. Guo Y, Lip GYH, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol 2012;60:2263–70. https://doi.org/10.1016/j.jacc.2012.04.063
32. Schnabel RB, Larson MG, Yamamoto JF et al. Relation of multiple inflammatory biomarkers to incident atrial fibrillation. Am J Cardiol 2009;104:92–6. https://doi.org/10.1016/j.amjcard.2009.02.053
33. Rudolph V, Andrié RP, Rudolph TK et al. Myeloperoxidase acts as a profibrotic mediator of atrial fibrillation. Nat Med 2010;16:470–4. https://doi.org/10.1038/nm.2124
34. Li J, Solus J, Chen Q et al. Role of inflammation and oxidative stress in atrial fibrillation. Heart Rhythm 2010;7:438–44. https://doi.org/10.1016/j.hrthm.2009.12.009
35. Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br J Clin Pharmacol 2013;75:645–62. https://doi.org/10.1111/j.1365-2125.2012.04374.x
36. Bloomer RJ, Larson DE, Fisher-Wellman KH, Galpin AJ, Schilling BK. Effect of eicosapentaenoic and docosahexaenoic acid on resting and exercise-induced inflammatory and oxidative stress biomarkers: a randomized, placebo controlled, cross-over study. Lipids Health Dis 2009;8:36. https://doi.org/10.1186/1476-511X-8-36
37. Ebrahimi M, Ghayour-Mobarhan M, Rezaiean S et al. Omega-3 fatty acid supplements improve the cardiovascular risk profile of subjects with metabolic syndrome, including markers of inflammation and auto-immunity. Acta Cardiol 2009;64:321–7. https://doi.org/10.2143/AC.64.3.2038016
38. Harada M, Van Wagoner DR, Nattel S. Role of inflammation in atrial fibrillation pathophysiology and management. Circ J Off J Jpn Circ Soc 2015;79:495–502. https://doi.org/10.1253/circj.CJ-15-0138
39. Oppedisano F, Mollace R, Tavernese A et al. PUFA supplementation and heart failure: effects on fibrosis and cardiac remodeling. Nutrients 2021;13:2965. https://doi.org/10.3390/nu13092965
40. Van Wagoner DR. Oxidative stress and inflammation in atrial fibrillation: role in pathogenesis and potential as a therapeutic target. J Cardiovasc Pharmacol 2008;52:306–13. https://doi.org/10.1097/FJC.0b013e31817f9398
41. Oppedisano F, Macrì R, Gliozzi M et al. The anti-inflammatory and antioxidant properties of n-3 PUFAs: their role in cardiovascular protection. Biomedicines 2020;8:306. https://doi.org/10.3390/biomedicines8090306
42. Liao C-H, Akazawa H, Tamagawa M et al. Cardiac mast cells cause atrial fibrillation through PDGF-A-mediated fibrosis in pressure-overloaded mouse hearts. J Clin Invest 2010;120:242–53. https://doi.org/10.1172/JCI39942
43. Samman Tahhan A, Sandesara PB, Hayek SS et al. Association between oxidative stress and atrial fibrillation. Heart Rhythm 2017;14:1849–55. https://doi.org/10.1016/j.hrthm.2017.07.028
