Thrombus is the main finding in most patients with acute coronary syndrome (ACS), the type of which potentially impacts the end result of the interventional procedure in terms of no reflow and edge dissection. Optical coherence tomography (OCT) is considered a precise intra-vascular tool to image thrombi and characterise its properties. We aimed to study the impact of thrombus type, as defined by OCT, on procedural outcome in ACS patients. In this retrospective study we enrolled 100 patients who were treated by percutaneous coronary intervention (PCI) with the guidance of OCT. We recorded demographic and clinical data of the whole studied cohort. Angiographic details and procedural data were noted. Baseline OCT study was performed before intervention and repeated post-intervention. Plaque characterisation was identified, and thrombi were defined as red or white thrombi. Acute angiographic outcome was defined with special emphasis on no reflow.
Male patients and ST-elevation myocardial infarction (STEMI) patients more often had white thrombi (58.1% and 71.2%, respectively). Cases with red thrombi had longer pain duration, which was statistically significant. Edge dissection was more frequent with red thrombus, 44.7% versus 32.1% with white thrombus, but the difference is not statistically significant, while 17% of patients with white thrombi were complicated by no-reflow phenomenon versus only 4.3% in patients with red thrombi, and this difference was statistically significant.
In conculsion, OCT-guided PCI is feasible and safe in ACS settings. OCT-guided intra-procedural differentiation of thrombus type is potentially beneficial in predicting acute procedural outcome.
Introduction
Acute coronary events are commonly caused by plaque rupture, erosion and, infrequently, calcific nodules. In the majority of patients with acute coronary syndrome (ACS), occlusive or sub-occlusive thrombus on top of plaque deformation is the main angiographic finding. Resolving acute thrombotic occlusion remains the cornerstone step in restoring adequate coronary perfusion. Blind dealing with thrombi, depending only on angiography, may be an obstacle to optimal myocardial perfusion and increase in-hospital morbidity and mortality.1–4
In the past, intravascular ultrasound (IVUS) and, more recently, optical coherence tomography (OCT) were frequently used for percutaneous coronary intervention (PCI) optimisation. Pre-PCI intra-vascular imaging, paved the way to precisely assess lesion significance, plaque features, necessity of lesion preparation and proper stent choice regarding width and length.4–6
Frequency domain OCT, as compared with IVUS, has 10 times better axial resolution, weak penetration capability (1–2 mm) and requires clearance of luminal blood. IVUS utilises ultrasound waves, in contrast to OCT, which depends on infrared light with minimal wavelength (1–3 µm) yielding magnificently higher resolution, but without satisfactory tissue penetration. The wavelength of red blood corpuscle is much greater than OCT wavelength, so backscattering occurs if luminal blood is not cleared first, before starting pullback. The combination of fine resolution with minimal penetration allows smooth distinction between plaque and abluminal surface, promoting OCT to be more superior in luminal measurements and clarifying thrombus composition.5–7
Frequency-domain OCT gives the privilege of going through the pathophysiology of unstable plaque, to differentiate between plaque rupture and erosion. Thrombi were defined as protrusions into the vessel lumen, and characterised according to their signal characteristics. White thrombus was identified as a signal-rich, low-backscattering thrombus, while red thrombus was identified as high-backscattering protrusions, with signal-free shadowing.8–14
PCI success might be hindered by the presence of intra-coronary thrombi, which influences acute procedural success in terms of improving coronary flow, thrombus prolapse and distal edge dissection. Impact of thrombus morphology and histology on procedural outcome has not been clearly studied. Depending on the physical characteristics of OCT, it is the most expected to precisely clarify thrombus type and study its impact on outcome.
We aimed to study the impact of thrombus type as defined by OCT on procedural outcome in ACS patients.
Method
Between January and June 2022, 760 cases of myocardial infarction were admitted to our centre, an invasive strategy including primary PCI was adopted in 524 patients. Of these, 131 patients underwent OCT-guided interventions based on operator experience and availability of probes; however, 31 patients were excluded either due to inappropriate image acquisition or due to excessive percutaneous transluminal coronary angioplasty (PTCA) with possible thrombus deformation. The remaining 100 patients met our inclusion criteria and were enrolled retrospectively. The exclusion criteria were: end-stage nephropathy, post-coronary artery bypass graft (CABG) patients, poor OCT image and patients with totally occlusive thrombi, even after wiring and thrombus aspiration. Written consent was achieved from every patient before participation.
Study population
Full history taking and precise clinical examination were done for all subjects. ST-elevation myocardial infarction (STEMI) was defined as typical chest pain for 30 minutes at least, arrival to centre with primary PCI facilities within 24 hours from onset of chest pain, ST-segment elevation >0.1 mV in two or more contiguous leads or newly detected left bundle-branch block. Non-ST-elevation myocardial infarction (NSTEMI) was defined as prolonged chest pain with positive cardiac biomarkers without ST-segment elevation. We defined the culprit vessel by electrocardiogram (ECG) criteria, or during angiogram as thrombus-containing vessel.13
During hospital course, serum creatinine was serially withdrawn. Duration of hospital course was recorded and early post-PCI negative outcomes were mentioned. Patients were followed in outpatient clinic for six months to detect major adverse cardiovascular events (MACE).
Patient preparation and OCT procedure
Antithrombotics were administered matching the latest universal guidelines. Drug-eluting stents were deployed. Lesion measurements were done in two orthogonal views and end-diastolic frames were selected, after giving at least 200 µg of nitrates. Post-OCT study, thrombotic lesions were categorised according to thrombus type into white and red thrombi.
A widely used frequency domain OCT system (Ilumien System, Inc., St. Jude Medical, USA) and a 0.014 inch wire (Image Wire, St. Jude Medical, USA) were used. Motorised wire pull-back at 10 mm/s was done during contrast injection. OCT measurements were mentored by an OCT specialist.2,15
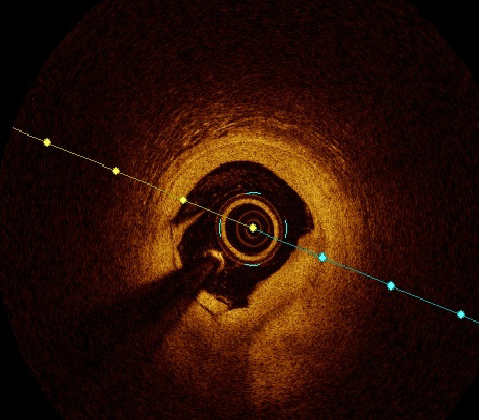
Plaque morphology was defined through every frame; plaque rupture was defined as discontinuity of the fibrous capsule with plaque cavitary formations. Plaque erosion was defined as irregular lumen with thrombosis overlying intact cap. Thrombus was categorised to red and white thrombi according to composition, attenuation degree and backscattering. White thrombus was defined as a signal-rich, low-backscattering mass, while red thrombus was defined as high-backscattering protrusions inside the lumen, with signal-free shadowing as shown in figures 1 and 2.2,16,17
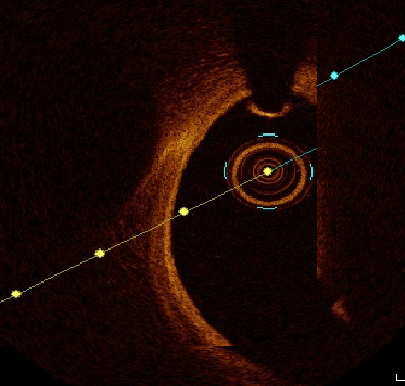
The OCT analysis included luminal areas at the proximal and distal referenced segments, minimal lumen areas (MLA), minimal stent area (MSA). MSA exceeding 90% was considered optimal and ≥80% was acceptable. Dissection was defined as disruption of luminal intimal surface at the stent edges, and tissue prolapse was defined as protrusion of tissue through stent struts. Thrombolysis in Myocardial Infarction (TIMI) flow grade was recorded with special emphasis on occurrence of no reflow.11–14,18
No reflow is defined as inadequate tissue perfusion through a given segment of the coronary circulation without angiographic evidence of major vessel obstruction.19
The management protocol of no-reflow phenomenon was primarily pharmacological through the intra-coronary injection of glycoprotein IIb/IIIa inhibitors, verapamil, sodium nitroprusside, adenosine and, infrequently, epinephrine.20
It is worth mentioning that thrombus identification by OCT is highly subjective and operator dependent.21
Statistical analysis
Statistical testing was done using the Statistical Package for Social Sciences (SPSS) version 20. Continuous variables were presented as mean ± standard deviation (SD). Categorical variables were presented as numbers and percentages. Kolmogorov-Smirnov test for normal distribution was used to delineate between parametric and non-parametric data. Analysis of variance (ANOVA) test was used to assess relations between different variables. Univariate and multi-variate analysis was done. For all tests, a p value less than 0.05 was considered a significant difference.
Results
Demographic data and risk factors
Table 1. Demographics and risk profile
| N=100 | Number | % |
|---|---|---|
| Male gender Female gender |
86 14 |
86 14 |
| Smokers | 79 | 79 |
| Hypertension | 60 | 60 |
| Diabetes mellitus | 34 | 34 |
| Hyperlipidaemia | 48 | 48 |
| Peripheral vascular disease | 0 | 0 |
| Cerebrovascular accident | 6 | 6 |
| Previous MI | 7 | 7 |
| Previous PCI | 23 | 23 |
| Mean ± SD | Range | |
| Age, years | 53.3 ± 11.3 | 28–85 |
| Duration of symptoms, hours | 9.7 ± 8.6 | 2–48 |
| Key: MI = myocardial infarction; PCI = percutaneous coronary intervention; SD = standard deviation | ||
One hundred patients were enrolled in this study. Patients’ demographic and clinical data are summarised in table 1. The mean age of studied patients was 53.3 years with males representing 86% of the studied cohort. Mean duration of chest pain was 9.7 hours. The most encountered risk factors were: cigarette smoking (79%), hypertension (60%), hyperlipidaemia (48%) and diabetes (34%), respectively. Six subjects had previously experienced cerebrovascular stroke.
Culprit lesion characteristics
Most of the patients in this study were STEMI patients (66%), two patients had acute pulmonary oedema and one patient was in cardiogenic shock. Of culprit lesions, 67% showed plaque rupture versus 33% with plaque erosion. Culprit vessel showed white thrombus in 53 subjects while 47 patients had red thrombus. No reflow was managed successfully using intra-coronary medications in 11 patients. Culprit lesion features are shown in table 2.
Procedural outcome
Drug-eluting stents were implanted in all subjects. Culprit vessel was left anterior descending (LAD) 73%, right coronary artery (RCA) 18%, left circumflex (LCx) 10%, left main trunk 3%, and diagonal 3%. OCT study post-intervention revealed mean stent expansion of 93.2% and mean MSA of 8 mm2. Limiting edge dissection was detected in six patients, tissue prolapse encountered in 38 cases and relevant mal-apposition necessitating optimisation in 56 patients (table 3).
Table 2. Culprit lesion morphology by optical coherence tomography (OCT)
| N=100 | Number | % | 95%CI |
|---|---|---|---|
| STEMI cases NSTEMI cases |
66 34 |
66 34 |
55 to 75% 24 to 44% |
| No-reflow phenomenon | 11 | 11 | 5 to 18% |
| Plaque rupture group | 67 | 67 | 56 to 76% |
| Plaque erosion group | 33 | 33 | 23 to 43% |
| Fibrous plaque | 24 | 24 | |
| Calcific plaque | 12 | 12 | |
| Necrotic fibrous plaque | 64 | 64 | |
| Thrombus type | |||
| Red thrombi | 47 | 47 | 36 to 57% |
| White thrombi | 53 | 53 | 42 to 63% |
| Mean ± SD | Range | ||
| Reference vessel diameter, mm | 3.3 ± 0.5 | 2.2–4.7 | |
| Lesion dimensions, mm | 1.52 ± 0.3 | 0.9–3 | |
| MLA in mm2 | 2.65 ± 1.0 | 0.8–8 | |
| Key: CI = confidence interval; MLA = minimal luminal área; NSTEMI = non-ST-elevation myocardial infarction; SD = standard deviation; STEMI = ST-elevation myocardial infarction | |||
Table 3. OCT study post-intervention
| N=100 | Number | % |
|---|---|---|
| Edge dissection | 6 | 6 |
| Tissue prolapse | 38 | 38 |
| Significant mal-apposition | 56 | 56 |
| Mean ± SD | Range | |
| Stent expansion, % | 93.2 ± 4.5 | 86–114 |
| Final MSA, mm2 | 8.0 ± 2.7 | 3.7–15.8 |
| Key: MSA = minimal stent area; SD = standard deviation | ||
White thrombus versus red thrombus
The studied cohort was categorised according to thrombus type to two groups: white thrombus (53%) versus red thrombus (47%). Both groups were compared according to demographic and clinical data as shown in table 4. White thrombus was found to be significantly more evident (58.1%) among male patients (p=0.009). Younger age at presentation was significantly related to white thrombi, while smokers only showed a trend for that. There is no statistically relevant difference regarding thrombus type among hypertensive, diabetic, or dyslipidaemia patients. White thrombi were significantly evident in STEMI patients (71.2%, p=0.000 with odds ratio 11.5) while in NSTEMI patients, white thrombi represented a minority. Cases with red thrombi had longer pain duration before presentation as compared with white thrombi, which was significant statistically (13.7 ± 9.4 vs. 6 ± 4.6 hours) as shown in table 4.
Table 4. Comparison between demographic and clinical variables as regards thrombus type
| Red thrombus Number (%) |
White thrombus Number (%) |
p value | |
|---|---|---|---|
| Male gender N=86 | 36 (41.9) | 50 (58.1) | 0.009 |
| Young (<55.3 years) N=50 | 17 (34) | 33 (66) | 0.009 |
| Smoker N=79 | 33 (41.8) | 46 (58.2) | 0.04 |
| Hypertensive N=60 | 32 (53.3) | 28 (46.7) | 0.1 |
| Diabetic N=34 | 18 (52.9) | 16 (47.1) | 0.3 |
| Dyslipidaemic N=48 | 26 (54.2) | 22 (45.8) | 0.1 |
| STEMI N=66 | 19 (28.8) | 47 (71.2) | |
| NSTEMI N=34 | 28 (82.4) | 6 (17.6) | 0.000 |
| Red thrombus Mean ± SD |
White thrombus Mean ± SD |
||
| Pain time in hours | 13.7 ± 9.4 | 6 ± 4.6 | 0.000 |
| Key: NSTEMI = non-ST-elevation myocardial infarction; SD = standard deviation; STEMI = ST-elevation myocardial infarction | |||
Composite negative procedural outcome in relation to thrombus type
Thrombus prolapse shows no statistically significant difference between both types of thrombi. Edge dissection was noticed more frequently with red thrombus 44.7% versus 32.1% among cases with white thrombus and the difference is not statistically significant. No-reflow phenomenon complicated 17% of patients with white thrombi versus only 4.3% of patients with red thrombi, and this difference was statistically significant. Collectively, a higher percentage of combined negative outcomes was witnessed among cases of red thrombi, but with no statistical significance (table 5).
Table 5. Comparison between type of thrombus as regards negative outcomes detected by OCT
| Red thrombus Number (%) |
White thrombus Number (%) |
p value | |
|---|---|---|---|
| Prolapse | 3 (6.4) | 3 (5.7) | 0.8 |
| Dissection | 21 (44.7) | 17 (32.1) | 0.1 |
| No reflow | 2 (4.3) | 9 (17) | 0.04 |
| Combined negative outcome | 23 (48.9) | 23 (43.3) | 0.5 |
Table 6. Logistic-regression model of thrombus type and the no-reflow phenomenon as a negative outcome during intervention
| No reflow | Wald | p | ||
|---|---|---|---|---|
| Exp(B) | 95%CI | |||
| Thrombus type (Red as having risk) |
0.22 | 0.05 to 1.1 | 3.5 | 0.059 |
| p>0.05 not significant Key: CI = confidence interval |
||||
Confirming the previous data in table 5, a logistic-regression model was conducted supporting that no reflow was not statistically related to red thrombi as compared with white thrombi with an odds ratio of 0.22, as shown in table 6.
Six-month follow-up
Recruited subjects were followed for six months in outpatient departments, no MACE were encountered in terms of cardiovascular-related mortality, myocardial infarction or target vessel re-intervention, apart from minor bleeding in a single subject and two patients’ admission with decompensated heart failure.
Discussion
The essential target of this study was to check the feasibility of OCT guidance in differentiating red and white thrombi within culprit lesions of ACS, thereafter, which type is more related to negative procedural outcomes such as no reflow, edge dissection, thrombus prolapse and their combined end point.
Most of the ACS patients in our study were presenting with STEMI (n=66). Plaque rupture was encountered in 77% of patients, where it represented 83% of STEMI cases, while plaque erosion represented 64% of NSTEMI patients. This finding is considered a rehearsal of what Guagliumi et al., Fang et al., and Dai et al. found in their studies.22–24
Before discussing our targeted differentiation of thrombus types to red and white, a very important clarification has to be done, because of which a lot of ACS patients were not recruited to our study. OCT-guided thrombus and plaque analysis was done only if at least TIMI flow ≥2 was achieved after culprit lesion wiring ± thrombus aspiration, but not after PTCA, fearing that PTCA may alter plaque morphology or add to negative outcomes as no reflow before our analysis. This pathway was also done by Amabile et al. when they studied thrombus burden by OCT before and after deferral of PCI in STEMI patients in 2014.25
White thrombus was more evident in male gender (58.1%) and also in young age (<53 years, our mean age) whereas smokers only trended for that. This can be attributed to early time of presentation in young male smoker patients who are not preconditioned to ischaemia at the time of presentation, when the thrombus is mainly formed of platelets, before fibrin meshwork is well established and red blood cells profoundly settle in the clot. Other atherosclerotic risk factors such as hypertension, diabetes or dyslipidaemia were not statistically different between the two types of thrombi.
White thrombi were consistently present in STEMI patients (71.2% of STEMI patients) but they represented a minority in NSTEMI patients, while the reverse was encountered with red thrombi. The group of patients with red thrombi had a longer duration of pain before presentation (13.7 ± 9.4 hours) compared with the group with white thrombi (6 ± 4.6 hours), which may be a dart to our point that fibrin mesh maturation and red blood cell settlement present after a longer time.
Despite a paucity of data about thrombus type in relation to different demographic, clinical and angiographic presentations, shades of our data were echoed in a review of literature published by Khandkar et al. in 2021 about the mechanistic differences among ACS patients, how to detect by imaging (where OCT surely was recommended as the best) and how this can affect the targeted procedure plan.26
As for acute procedural negative outcomes, our study focused on no reflow, thrombus prolapse and dissection. White thrombi were found to be strongly related to occurrence of no reflow, which happened in 17% of white thrombi patients versus only 4.3% of red thrombi patients. Again, this was not stated as solidly as we state before, but suggested by Khandkar et al. by relating ruptured fibrous cap to STEMI patients to white thrombi, then relating no reflow to STEMI patients.26
Logically, if no-reflow phenomenon is mostly due to micro-thrombi dislodgement to distal circulation after PTCA or stent deployment, as stated in almost all literature discussing this phenomenon, this shall happen more commonly with the platelet-rich white thrombi than with the fibrin and red blood cell-rich red thrombi. This could also explain the good response of no reflow to intracoronary glycoprotein IIb/IIIa inhibitors to stop platelet aggregation and white thrombus propagation.20,22
Regarding thrombus prolapse, there was no significant difference between the two types of thrombi, while dissection was encountered more with red thrombi patients but without statistical significance. Also, the combined negative outcome showed no difference between the two thrombi types.
This study is not a limitation-free study, being a non-randomised study, selection bias and other forms of bias cannot be excluded. Moreover, the study was not empowered to detect differences in MACE or other clinical outcomes. So, larger randomised clinical studies are needed to clarify these gaps of evidence. Despite these limitations, we do believe that our data are potentially adding to the body of evidence supporting the use of OCT as the best intra-vascular imaging modality to optimise acute procedural outcomes in ACS patients.
Conclusion
OCT-guided PCI is feasible and safe among patients with ACS. OCT-guided intra-procedural differentiation of thrombus type is potentially beneficial in predicting acute procedural outcome.
Key messages
- Optical coherence tomography (OCT) is feasible in setting of acute coronary syndrome (ACS) when used by experienced hands
- OCT can perfectly optimise angiographic outcome post-percutaneous coronary intervention (PCI)
- White thrombus is a major risk factor of no reflow
- Anticipation of no reflow is possible with intravascular imaging
Conflicts of interest
None declared.
Funding
None.
Study approval
The study had ethical approval number FMASU R 162 2022.
References
1. Virmani R, Burke AP, Kolodgie FD et al. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol 2000;20:1262–75. https://doi.org/10.1161/01.ATV.20.5.1262
2. Jia H, Aguirre AD, Abtahian F et al. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J Am Coll Cardiol 2013;62:1748–58. https://doi.org/10.1016/j.jacc.2013.05.071
3. O’Donoghue M, Braunwald E, Boden WE et al. Early invasive vs conservative treatment strategies in women and men with unstable angina and non-ST segment elevation myocardial infarction: a meta-analysis. JAMA 2008;300:71–80. https://doi.org/10.1001/jama.300.1.71
4. Cannon CP, Demopoulos LA, Weintraub WS et al.; TACTICS (Treat Angina with Aggrastat and Determine Cost of Therapy with an Invasive or Conservative Strategy) – Thrombolysis in Myocardial Infarction 18 Investigators. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med 2001;344:1879–87. https://doi.org/10.1056/NEJM200106213442501
5. FRagmin and Fast Revascularisation during InStability in Coronary artery disease (FRISC II)Investigators. Invasive compared with non-invasive treatment in unstable coronary-artery disease: FRISC II prospective randomised multicentre study. Lancet 1999;354:708–15. https://doi.org/10.1016/S0140-6736(99)07349-3
6. Fox KA, Henderson RA, Poole-Wilson PA et al.; Randomized Intervention Trial of unstable Angina Investigators. Interventional versus conservative treatment for patients with unstable angina or non-ST-elevation myocardial infarction: the British Heart Foundation RITA 3 randomized trial. Lancet 2002;360:743–51. https://doi.org/10.1016/S0140-6736(02)09894-X
7. Nakazawa G, Joner M, Finn AV et al. Delayed arterial healing and increased late stent thrombosis at culprit sites after drug-eluting stent placement for acute myocardial infarction patients: an autopsy study. Circulation 2008;118:1138–45. https://doi.org/10.1161/CIRCULATIONAHA.107.762047
8. Yamamoto M, Okamatsu K, Inami S et al. Relationship between neointimal coverage of sirolimus-eluting stents and lesion characteristics: a study with serial coronary angioscopy. Am Heart J 2009;158:99–104. https://doi.org/10.1016/j.ahj.2009.04.016
9. Kramer MC, Rittersma SZ, de Winter RJ et al. Relationship of thrombus healing to underlying plaque morphology in sudden coronary death. J Am Coll Cardiol 2010;55:122–32. https://doi.org/10.1016/j.jacc.2009.09.007
10. Chieffo A, Caussin C, latib A et al. A prospective, randomized trial of intravascular-ultrasound guided compared to angiography guided stent implantation in complex coronary lesions: the AVIO trial. Am Heart J 2013;165:65–72. https://doi.org/10.1016/j.ahj.2012.09.017
11. Kang SJ, Park GM, Cho YR et al. Intravascular ultrasound predictors for edge restenosis after newer generation drug-eluting stent implantation. Am J Cardiol 2013;111:1408–14. https://doi.org/10.1016/j.amjcard.2013.01.288
12. Ino Y, Matsuo Y, Kubo T et al. Optical coherence tomography predictors for edge restenosis after everolimus-eluting stent implantation. Circ Cardiovasc Interv 2016;9:e004231. https://doi.org/10.1161/CIRCINTERVENTIONS.116.004231
13. Prati F, Burzotta F, Romagnoli E et al. Clinical impact of OCT findings during PCI: the CLI-OPCI II study. JACC Cardiovasc Imaging 2015;8:1297–305. https://doi.org/10.1016/j.jcmg.2015.08.013
14. Prati F, Regar E, Mintz GS et al. Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur Heart J 2010;31:401–15. https://doi.org/10.1093/eurheartj/ehp433
15. Witzenbichler B, Weisz G, Maehara A et al. Relationship between intravascular ultrasound guidance and clinical outcomes after drug-eluting stents: the Assessment Of Dual Antiplatelet Therapy with Drug-Eluting Stents (ADAPT-DES) study. Circulation 2014;129:463–70. https://doi.org/10.1161/CIRCULATIONAHA.113.003942
16. Ali ZA, Généreux P, Maehara A et al. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): a randomized controlled trial. Lancet 2016;388:2618–28. https://doi.org/10.1016/S0140-6736(16)31922-5
17. Liu J, Mintz GS, Maehara A et al. An integrated TAXUS IV, V, and VI intravascular ultrasound analysis of the predictors of edge restenosis after bare metal or paclitaxel-eluting stents. Am J Cardiol 2009;103:501–06. https://doi.org/10.1016/j.amjcard.2008.10.010
18. Prati F, Guagliumi G, Mintz GS et al. Expert review document part 2: methodology, terminology and clinical applications of optical coherence tomography for the assessment of interventional procedures. Eur Heart J 2012;33:2513–20. https://doi.org/10.1093/eurheartj/ehs095
19. Rezkalla SH, Kloner RA. No-reflow phenomenon. Circulation 2002;105:656–62. https://doi.org/10.1161/hc0502.102867
20. Ramjane K, Han L, Jin C. The diagnosis and treatment of the no-reflow phenomenon in patients with myocardial infarction undergoing percutaneous coronary intervention. Exp Clin Cardiol 2008;13:121–8. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2586408/
21. Kaivosoja T, Liu S, Dijkstra J et al. Comparison of visual assessment and computer image analysis of intracoronary thrombus type by optical coherence tomography. PLoS One 2018;13:e0209110. https://doi.org/10.1371/journal.pone.0209110
22. Guagliumi G, Capodanno D, Saia F et al. Mechanisms of atherothrombosis and vascular response to primary percutaneous coronary intervention in women versus men with acute myocardial infarction: results of the OCTAVIA study. JACC Cardiovasc Interv 2014;7:958–68. https://doi.org/10.1016/j.jcin.2014.05.011
23. Fang C, Dai J, Zhang S et al. Culprit lesion morphology in young patients with ST-segment elevated myocardial infarction: a clinical, angiographic and optical coherence tomography study. Atherosclerosis 2019;289:94–100. https://doi.org/10.1016/j.atherosclerosis.2019.08.011
24. Dai J, Xing L, Jia H et al. In vivo predictors of plaque erosion in patients with ST-segment elevation myocardial infarction: a clinical, angiographical, and intravascular optical coherence tomography study. Eur Heart J 2018;39:2077–85. https://doi.org/10.1093/eurheartj/ehy101
25. Amabile N, Hammas S, Fradi S et al. Intra-coronary thrombus evolution during acute coronary syndrome: regression assessment by serial optical coherence tomography analyses. Eur Heart J Cardiovasc Imaging 2015;16:433–40. https://doi.org/10.1093/ehjci/jeu228
26. Khandkar C, Madhavan MV, Weaver JC et al. Atherothrombosis in acute coronary syndromes – from mechanistic insights to targeted therapies. Cells 2021;10:865. https://doi.org/10.3390/cells10040865
