Primary percutaneous coronary intervention (PPCI) remains the gold-standard treatment for ST-elevation myocardial infarction (STEMI). Femoral arterial access for the procedure may be an ideal option in patients who are haemodynamically unwell. However, it is associated with rare, but life-threatening, complications such as perforation, leading to retroperitoneal haemorrhage. We present the case of a man in his 50s, admitted with cardiac arrest secondary to inferolateral STEMI. Successful PPCI was performed via right femoral artery, with access gained under ultrasound guidance. However, the patient deteriorated and was diagnosed to have a retroperitoneal haematoma secondary to femoral artery perforation. Additional arterial access via left brachial artery was obtained, and a covered stent was deployed successfully in the right femoral artery with satisfactory haemostasis. The patient recovered successfully and was discharged two weeks later. Early recognition of such complications is imperative to adequate management and percutaneous treatment is a viable option for such situations, in comparison with open surgical repair.
Background
Radial artery is the preferred route of access for percutaneous coronary intervention (PCI) as the femoral artery is associated with a greater incidence of bleeding complications.1–5 Often such complications require open surgical repair.4,6 However, percutaneous treatment with covered stents may represent an alternate option.
Case presentation
A man in his 50s, fit and well and on no routine medications, developed chest pain and posterior ST-elevation myocardial infarction (STEMI) with 2 mm ST-elevation in posterior leads V7, 8 and 9, and 2 mm ST-depression in leads V2 and 3, complicated by cardiogenic shock and out-of-hospital cardiac arrest requiring two defibrillator shocks with a downtime of 20 minutes. Aspirin 300 mg and ticagrelor 180 mg were administered via a nasogastric tube. Since both radial pulses were non-palpable, arterial access via the right femoral artery was gained using ultrasound and fluoroscopy guidance, and a 6-French (Fr) sheath was introduced for emergent angiography, revealing thrombotic occlusions of the left circumflex (LCx) coronary artery and right coronary artery (RCA). Eight thousand units of unfractionated heparin were administered intra-arterially. The LCx lesion was successfully predilated and then stented with a 3.0 × 24 mm biolimus drug-eluting stent (Biomatrix, Biosensors Europe, SA, Switzerland) and RCA was successfully treated with a 3.5 × 19 mm biolimus drug-eluting stent (Biomatrix, Biosensors Europe, SA, Switzerland).
 |
 |
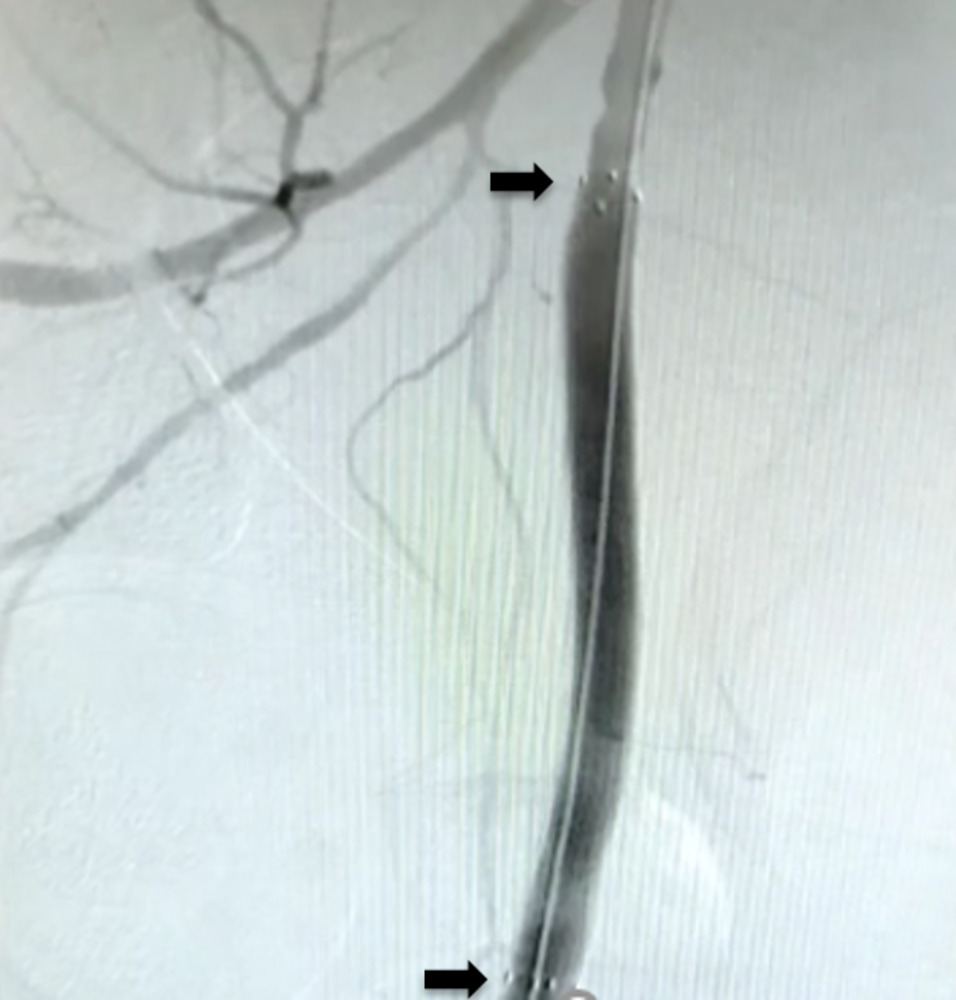 |
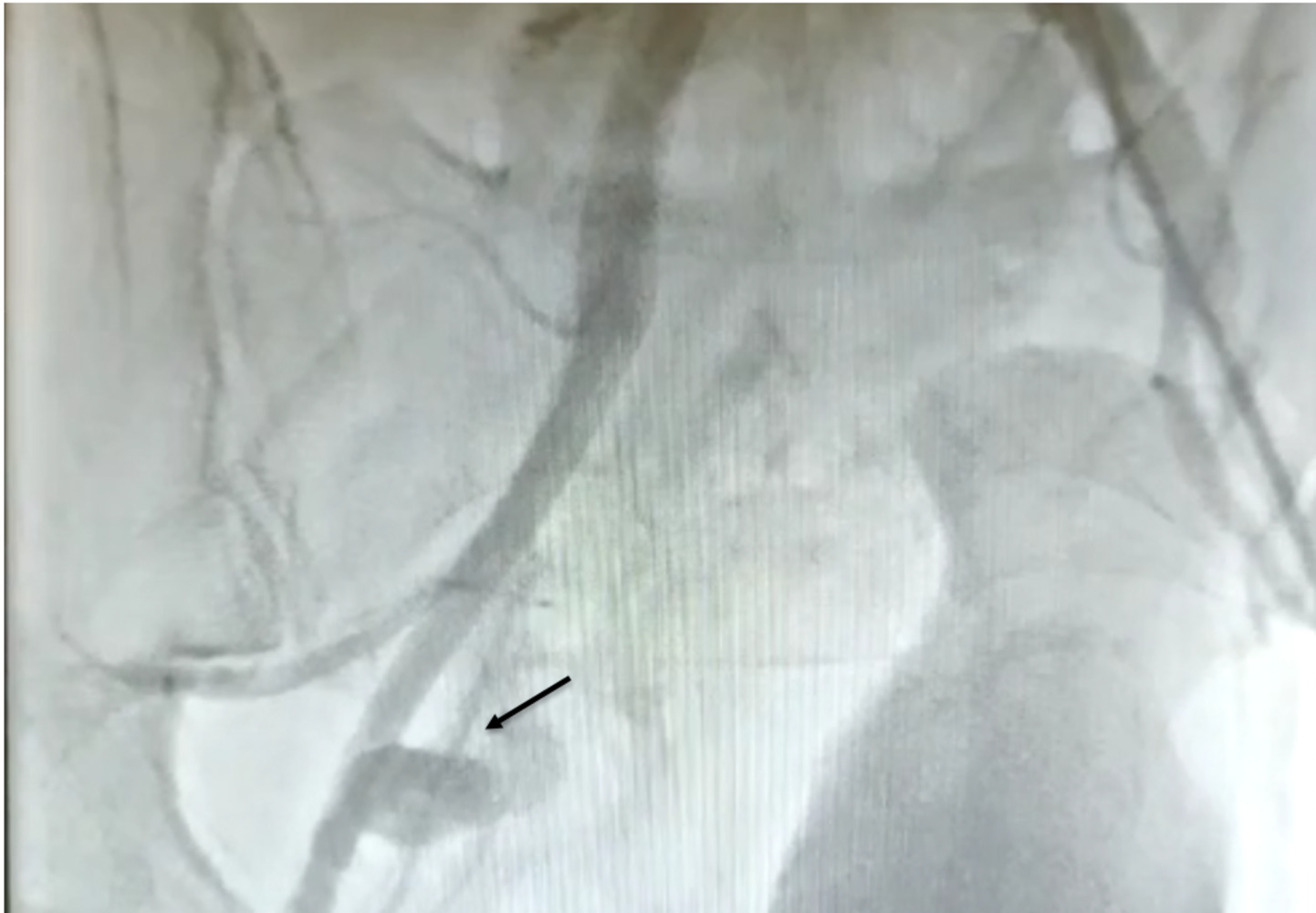
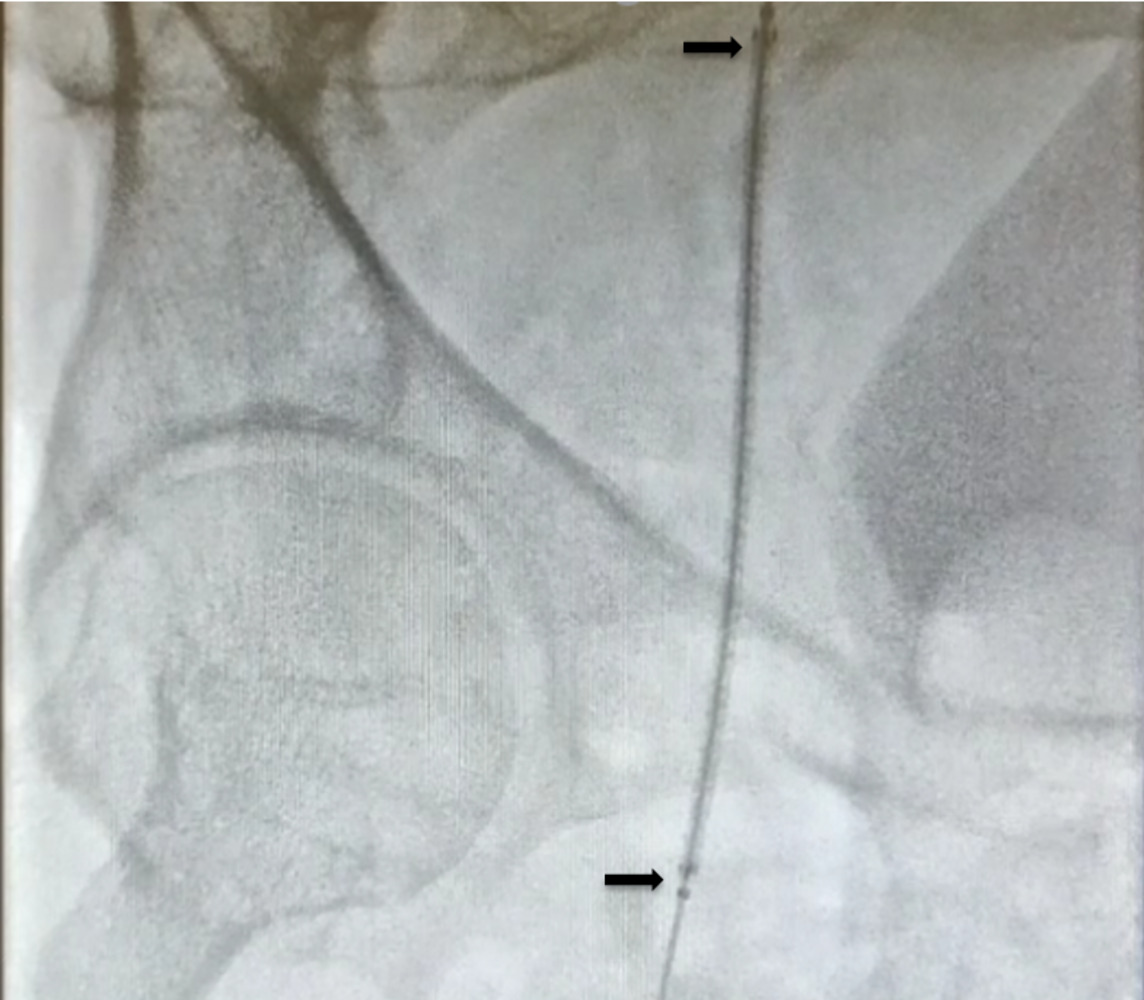
The patient remained haemodynamically unstable. A right femoral haematoma was noted at the site of the arterial access. Manual pressure was applied and an angioseal (Terumo, Europe, NV) was attempted to be deployed. However, we were unable to advance it over the guidewire; therefore, the sheath was removed once activated clotting time was less than 150 ms and manual pressure applied. Fluoroscopy showed blunting of the right bladder wall. The patient was immediately resuscitated with intravenous fluids and blood products, but remained too haemodynamically unstable to transfer for urgent computed tomography (CT) angiogram. Therefore, an ultrasound-guided left femoral arterial (LFA) access was gained and femoral angiography performed, revealing extravasation of contrast into the pelvis, proximal to the common femoral artery (CFA) bifurcation (figure 1). The patient remained too unstable to transfer to the vascular department for open surgical repair, located at a different site. Therefore, a covered stent was attempted to be delivered via LFA to control the bleeding. Unfortunately, the acute angle between the common iliac arteries did not allow the passage of equipment. An ultrasound-guided, left brachial access was gained and a 8-Fr sheath was introduced. A multi-purpose (MPA) guide catheter (Medtronic, Minneapolis, MN) was advanced over a 0.035 inch guidewire to the right common femoral artery and a Viabahn® 8 × 100 mm covered stent prosthesis (Gore, Barcelona, Spain) was delivered proximal to RFA bifurcation (figure 2). Subsequent angiography showed that satisfactory haemostasis was achieved (figure 3). A 6-Fr angioseal (Terumo, Europe, NV) was used to close the LFA arteriotomy, and manual pressure was used to achieve haemostasis at the left brachial artery access site. The patient subsequently made good recovery with intact neurological status. Transthoracic echocardiography showed inferior and posterior regional wall motion abnormalities, with an ejection fraction of 54%. He was continued on standard post-acute coronary event treatment and was discharged after two weeks without any further sequalae.
Discussion
Access via radial artery is preferred as the femoral artery is associated with greater bleeding complications.2–4,7 Nevertheless, it may represent the only option in patients with poor upper limb pulses.8 Retroperitoneal bleeding remains a rare but important complication of femoral arterial access and may lead to prolonged hospital stay and significant morbidity and mortality.7,9-12 Risk factors include female gender, low body weight, advanced age, anticoagulation, recurrent intervention and high stick entry location of the sheath.6,12–14 Bleeding may occur at the arteriotomy site as well as higher up.6,11,15
Femoral arterial access via a micropuncture technique, using an initial 21-gauge introducer needle and 4-Fr sheath and then upgrading to 6-Fr sheath, is shown to reduce the rate of vascular complications.16 However, since the smaller lumen of the introducer needle results in much slower blood flow in comparison to standard systems, in situations where the blood pressure is low, there may be uncertainty regarding the needle accessing the true lumen of the artery.17 To avoid this, as well as the need for switching sheath sizes causing further delay, we opted to go with upfront 6-Fr size standard sheath. Micropuncture technique used with ultrasound guidance could potentially be more successful, as the latter is known to improve first pass rates and inadvertent venipuncture,8 thereby, ensuring true lumen access, in addition to early detection of such complications when follow-on femoral angiography is performed prior to sheath upgrade. However, the incidence of significant bleeding or complications was not significantly different when ultrasound guidance was used alone.8
Stable patients with contained bleeding may be treated with conservative therapy, involving fluid resuscitation and manual pressure. Unstable patients, in addition, may require percutaneous or open surgical repair to achieve haemostasis, although the latter is associated with higher morbidity and mortality.5–7
We managed our patient percutaneously. Our attempt via the left femoral artery proved unsuccessful due to the acute angle of the abdominal aortic bifurcation. We decided to attempt via the left brachial artery, which has a larger caliber than the radial artery and may accommodate larger sheath sizes for delivery of the required stents. We delivered and deployed a covered stent prosthesis, which successfully achieved haemostasis. A balloon tamponade was not attempted in the first instance as the clinical impression was that the bleeding site was probably significantly large, evident from the degree of haemodynamic instability and bleeding, and that this alone would not have been successful, and may have caused further delay and harm from uncontrolled bleeding. The patient recovered well and, on follow-up one month later, did not report any significant angina or limb ischaemia, and had good neurological recovery.
Conclusion
Retroperitoneal bleeding remains a major complication associated with femoral artery access in patients undergoing PCI. Percutaneous management represents a feasible option in unstable patients where expertise is available.
Key messages
- Retroperitoneal bleeding continues to be a rare but major complication of femoral arterial access for percutaneous coronary intervention
- High index of suspicion is critical to recognise such complications early on for timely management, especially in a patient with altered consciousness where usual symptoms, such as groin or abdominal pain, may not be reported. Micropuncture technique with ultrasound guidance and subsequent angiography may be helpful in early detection and management of such complications
- Percutaneous treatment of this complication with a covered stent is a feasible option, where facilities and expertise are available, and would avoid the need for open vascular repair, which represents a major surgical procedure
- Good communication and teamwork with appropriate specialities are key to optimal patient care and management of major procedural complications
Conflicts of interest
None declared.
Funding
None.
Patient consent
Consent for publication was obtained from the patient.
References
1. Ibanez B, James S, Agewall S et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2018;39:119–77. https://doi.org/10.5603/KP.2018.0041
2. Jolly SS, Yusuf S, Cairns J et al. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet 2011;377:1409–20. https://doi.org/10.1016/S0140-6736(11)60404-2
3. Valgimigli M, Gagnor A, Calabró P et al. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: a randomised multicentre trial. Lancet 2015;385:2465–76. https://doi.org/10.1016/S0140-6736(15)60292-6
4. Romagnoli E, Biondi-Zoccai G, Sciahbasi A et al. Radial versus femoral randomized investigation in ST-segment elevation acute coronary syndrome. J Am Coll Cardiol 2012;60:2481–9. https://doi.org/10.1016/j.jacc.2012.06.017
5. Kwok CS, Kontopantelis E, Kinnaird T et al. Retroperitoneal hemorrhage after percutaneous coronary intervention. Circ Cardiovasc Interv 2018;11:e005866. https://doi.org/10.1161/CIRCINTERVENTIONS.117.005866
6. Kumar AR, Rajiv A. Percutaneous treatment of severe retroperitoneal hematoma after percutaneous coronary intervention. J Cardiol Cardiovasc Med 2021;6:055–058. https://doi.org/10.29328/journal.jccm.1001119
7. Gündeş E, Aday U, Bulut M et al. Factors affecting treatment, management and mortality in cases of retroperitoneal hematoma after cardiac catheterization: a single-center experience. Postepy Kardiol Interwencyjnej 2017;13:218–24. https://doi.org/10.5114/aic.2017.70189
8. Jolly SS, AlRashidi S, d’Entremont MA et al. Routine ultrasonography guidance for femoral vascular access for cardiac procedures: the UNIVERSAL randomized clinical trial. JAMA Cardiology 2022;7:1110–18. https://doi.org/10.1001/jamacardio.2022.3399
9. Tiroch KA, Arora N, Matheny ME, Liu C, Lee TC, Resnic FS. Risk predictors of retroperitoneal hemorrhage following percutaneous coronary intervention. Am J Cardiol 2008;102:1473–6. https://doi.org/10.1016/j.amjcard.2008.07.039
10. Trimarchi S, Smith DE, Share D et al. Retroperitoneal hematoma after percutaneous coronary intervention: prevalence, risk factors, management, outcomes, and predictors of mortality: a report from the BMC2 (Blue Cross Blue Shield of Michigan Cardiovascular Consortium) registry. JACC Cardiovasc Interv 2010;3:845–50. https://doi.org/10.1016/j.jcin.2010.05.013
11. Farouque HMO, Tremmel JA, Raissi Shabari F et al. Risk factors for the development of retroperitoneal hematoma after percutaneous coronary intervention in the era of glycoprotein IIb/IIIa inhibitors and vascular closure devices. J Am Coll Cardiol 2005;45:363–8. https://doi.org/10.1016/j.jacc.2004.10.042
12. Vilke GM, Kass P. Retroperitoneal hematoma after femoral arterial catheterization. J Emerg Med 2015;49:338–9. https://doi.org/10.1016/j.jemermed.2015.05.002
13. Piper WD, Malenka DJ, Ryan TJ et al. Predicting vascular complications in percutaneous coronary interventions. Am Heart J 2003;145:1022–9. https://doi.org/10.1016/S0002-8703(03)00079-6
14. Wiley JM, White CJ, Uretsky BF. Noncoronary complications of coronary intervention. Catheter Cardiovasc Interv 2002;57:257–65. https://doi.org/10.1002/ccd.10307
15. Liu SY, Zeng B, Deng JB. Massive retroperitoneal hemorrhage secondary to femoral artery puncture: a case report and review of literature. Medicine (Baltimore) 2017;96:e8724. https://doi.org/10.1097/MD.0000000000008724
16. Ben-Dor I, Sharma A, Rogers T et al. Micropuncture technique for femoral access is associated with lower vascular complications compared to standard needle. Catheter Cardiovasc Interv 2021;97:1379–85. https://doi.org/10.1002/ccd.29330
17. Ben-Dor I, Bernardo NL, Satler LF, Pichard AD, Waksman R. Applying micropuncture access. When and how this technique is useful in large-sheath procedures. Cardiac Interventions Today 2012;September/October:53–7. Available from: https://citoday.com/articles/2012-sept-oct/applying-micropuncture-access
