Andreas Grüntzig, an ardent angiologist crafted an indeflatable sausage-shaped dual-lumen balloon-catheter, designed its delivery to the heart, launched minimally invasive coronary intervention and taught by beaming live demonstration. Subsequent advances are just incremental tweaks and tinkers around this fully formed framework from 1978. The near-immediate or instant feedback learning process by which the heart responds to any new invasive procedural variation facilitates each new change; be it drug-eluting stent, drug-coated balloon, or both in different combinations and permutations. Now with Grüntzig’s balloon armed with an antiproliferative drug, it could dominate the field once more, as he originally envisaged.
Introduction

The use of drug-coated balloon (DCB) in de novo coronary artery disease has seeped through into routine practice in recent years.1 Largely unnoticed by the mainstream community, ignored by multi-national device companies and rarely discussed at international meetings. Its development actually parallels that of first-generation drug-eluting stent (DES) from the early 2000s; pioneered, propagated and instructed by expert German operators.2,3 Its efficacy is proven for in-stent re-stenosis (ISR),4 small vessel disease,5,6 high-risk bleeder,7 and where stenting might be avoided, such as in Takayasu arteritis.8 It has been safely trialled in ST-elevation myocardial infarction (STEMI),9,10 side-branch involving left main,11–13 multi-vessel,14 large-vessel15 and calcified16,17 disease. Simply put, the whole spectrum of ischaemic heart disease is amenable to DCB, notwithstanding ectatic or aneurysmal coronary lesion that is too large to stent.18,19
DCB practice
Searching with the terms “DCB”, “coronary” and “de novo” in Google Scholar brings up publications reaching 100 per month in 2022, compared with 140 for DES. Although the current drive for DCB is mainly in Far Eastern and Nordic countries. However, the evidence-base and strut/polymer/drug technology for DES have matured and its price has been driven down by competition. DCB costs two to three times more than DES. With the realisation that the bulk of coronary interventions could be completed with DCB, it is conceivable that big players will soon enter the foray. Cordis Corporation, which implanted the first DES in 1999 and launched it in 2003, has just reportedly acquired a DCB being studied for de novo use for nearly £1 billion. Its first-generation DES was hamstrung by thick stent strut, polymer allergy, stent thrombosis and excess mortality after two years.20 It might lead again, using the same antiproliferative drug, this time delivered not with stent, but by balloon. Hence, leaving nothing behind, that is, no stent, no stent thrombosis.
In 2022 alone, over one million DCB-only or 20–25% of coronary interventions were undertaken in China. In the UK, DCB use was 8.1%, mainly for ISR but with a small de novo component, according to the British Cardiovascular Intervention Society (BCIS) national audit data from 2021/2022. If 70% of coronary cases are done with DCB in the UK, it would be an estimated £30 million market per annum in the next five to 10 years (see below for DCB vs. DES split). The DCB journey is akin to that of radial approach adoption, which is presently the dominant vascular access by diktat following a 25-year journey from fringe to the forefront. Each technique has its own performance bias. With radial access, the mortality benefit in acute coronary syndrome is only apparent in the above 70% high-volume operators.21,22 DCB is similar, and its quarter-century voyage reaches its zenith by 2028, as coronary intervention did in 2003, when the author started his training.23
The beginning
Percutaneous coronary transluminal angioplasty or plain-old balloon angioplasty (POBA) was first reported in The Lancet by Andreas Grüntzig (1939–85), a visionary German angiologist.24 He improved on Charles Dotter’s technique performing peripheral angioplasty with his patented double-lumen balloon-catheter. He forged his sausage-shaped balloon with polyvinyl chloride (PVC) plastic after consulting with Heinrich Hopff, a retired German organic chemist in Zurich.25 He designed a system with pressured-and-contrasted balloon indeflation, blood pressure transducing and radiographic-dye injection for visualisation to perform coronary angioplasty. This was with low-resolution cine-fluoroscopy without the replay button. He first tested his balloon on dogs, followed by patients undergoing coronary artery bypass grafting (CABG). It was a theatrical performance then, as it is today, when he imparted his technique broadcasted to a live audience starting on 7–10 August 1978; four such meetings took place in Zurich while he was there.25–27
POBA has an instant feedback for success or failure, with 10% abrupt vessel closure (AVC), which Grüntzig had a tough time explaining to seniors at his institution, who were less than sympathetic, culminating with him leaving Zurich for Atlanta in 1980; entering the US as a national treasure.23 He was offered a professorship and unlimited funding at Emory University, courtesy of a 100 million dollar grant from Coca Cola, which is headquartered at Atlanta.27 Grüntzig’s Lancet series describes five patients. One was a 44-year-old man with an undilatable calcified circumflex artery. Another man, Dölf Bachmann who was 38, coincidentally the same age as Grüntzig at the time, was symptom free from his proximal left anterior descending (LAD) POBA for 23 years. He was also Grüntzig’s first coronary patient treated on 16 September 1977.23 At 61 years of age, he had angina again with borderline pressure-wire fractional flow reserve of 0.84 and he had a bare-metal stent placed, which failed within two months. This was balloon dilated. He was well for another 14 years until 75 years old when he had a DES for recurrent ISR. One could speculate that he would have had fewer repeat procedures if Bernhard Meier, Grüntzig’s protégé had access to DCB. He detailed this man’s clinical course in his Andreas Grüntzig Lecture at the 35th European Society of Cardiology (ESC) Congress in London on 30 August 2015. Early adopters of POBA had a 6–10% failure rate necessitating emergency CABG.25,28,29 There was a learning curve in case selection and skilful lesion preparation. For instance, Geoffrey Hartzler’s institutional failure rate was under 1% after a decade’s practice, from 10%.28
Stent issues
DES is recommended by guidelines for STEMI.4 The CADILLAC (Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications) study, which was conducted over 20 years ago, seemingly confirmed the superiority of stenting.30 But this was on the basis of planned target-vessel revascularisation at six months, which was not clinically driven. Nevertheless, experience gained over the last two decades has uncovered some downsides to stenting in STEMI. These include no reflow from distal embolisation, causing microvascular obstruction that extends infarct size, as with side-branch occlusion.31,32 In the mid-LAD, which is replete with side branches, the septal and diagonal branches are vulnerable to occlude with stent optimisation from plaque or thrombotic shift. Conversely, stent mal-apposition could result from stent under-sizing from vasoconstriction during STEMI.33
With stenting in general, beyond nine months there is a continuous risk of ISR, from 5–15% within the year to a year-on-year rise to 25–30% in three to five years; this curve continues to climb, even with third-generation DES.20,34–37 The ideal stenting rate is probably around 30%, according to Bernhard Meier, as per law of diminishing return for POBA and bare-metal stent.36 Conjecturally, the same ratio holds true for DCB and DES. Target-vessel failure might partly be driven by vascular endothelial dysfunction that is exacerbated by the stent.38 There is also renewed interest in the coronary microvasculature.39 It plays a central role in coronary blood flow autoregulation, which explains the disconnect between epicardial coronary artery disease, symptoms and mortality in patients with stable disease,40,41 chronic kidney disease (CKD),42 and heart failure;43 where DES imparts salutary and no lasting benefit, nor does it impact survival in the unselected population.44
In another Andreas Grüntzig Lecture at the 42nd ESC Congress on 27 August 2022 in Barcelona, Javier Escaned cautioned that a third of daily coronary interventional practice now deals with stent failures. These are mainly caused by the metallic stent strut and neo-atheroma. Neither thinner strut nor abluminal biodegradable polymer coating offers durable outcomes, and the search for a bioresorbable stent remains elusive. Abbott’s Scaffold, a short-lived experiment with a bioresorbable poly-L-lactide strut, instead gave rise to the imaging-guided stenting approach. This involves extensive stenting for more complete plaque coverage, possibly contributing to further stent failures, as stenting length of over 40 mm is a potent predictor.34,45
Plaque stabilisation?
The shortcomings of DES might be avoided by DCB. The large SCAAR (Swedish Coronary Angiography and Angioplasty Registry)46 and other47,48 research registries in the mode of post-marketing surveillance have demonstrated long-term efficacy and safety of DCB in all-comers, including STEMI. What is remarkable is that the target-vessel failure curve flattens after three years, reminiscent of a ‘cure’. This is also observed in some randomised-controlled trials (RCTs) comparing DES and DCB.5,49 Not unlike that of POBA, in the author’s 20-year practice, only an 89-year-old woman re-presented for surveillance of her aortic stenosis. She had POBA 30 years prior in 1993 and has been angina free since. Perhaps this is because, without a stent-caged artery, POBA or DCB preserves vascular endothelial function,50,51 regresses atherosclerotic plaque,52 and, in an animal experiment, stabilises vulnerable plaque.53 The latter is being examined in the DEBuT-LRP (Intravascular Identification and Drug-Eluting Balloon Treatment of Vulnerable Lipid-Rich Plaques) study (NCT04765956).
Abrupt vessel closure
There is undoubtedly a learning curve with DCB harking back to the POBA era.24,28 It is clear that AVC is mostly thrombotic,54 and rarely is due to occlusive dissection.55 The former is uncommon with modern antiplatelet drugs pretreatment, while the latter requires stenting, and could be anticipated with practice, such that it becomes less frequent than acute stent thrombosis.1,55 Otherwise, ambulatory DCB practice would not be feasible.56 Pressure-wire guided DCB,57 and controlled plaque-modification,8 provide added confidence. Although the fear of AVC without stenting is real, be that as it may, advanced chronic-total occlusion (CTO) operators are quite content to leave a dissected vessel unstented as an investment procedure (Invest CTO PCI Trial: NCT04774913). On the other hand, stent thrombosis occurs in 0.8% (one in 125) and 1% of cases within 30 days and one year, respectively, according to the BCIS national audit data. There are patient and procedural factors. Technically, stent under expansion and mal-apposition are probably less common with current practice, with the increased awareness for these, and the plethora of imaging and calcium modification devices we have in our armamentarium. However, the reverse might be happening. Stent oversizing, aggressive post-dilatation or stenting into a diseased segment causes stent-edge dissection (SED) in up to 20% of cases, detectable with optical coherence tomography (OCT), which carries a 5% mortality within three months.58 This is because the resulting flap of tissue beyond the distal stent edge goes against the blood flow, extending the dissection, impeding blood circulation and precipitating subacute stent thrombosis (figure 1).
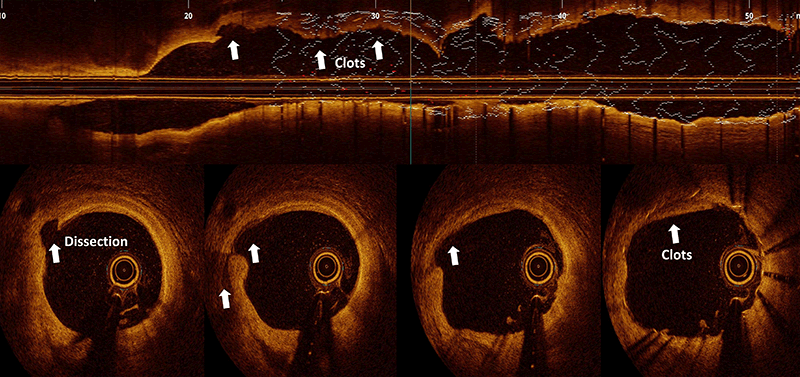
POBA without stenting could be achieved in over 95% of STEMI cases in RCTs.10,30–32 In the DANAMI-DEFER (Deferred versus Conventional Stent Implantation in Patients with ST-segment Elevation Myocardial Infarction) study,31 deferring stenting for three days (612 stenting and 603 defer), AVC in the second half of study occurred in one (<1%) case versus 11 (1.5%) cases indicating a learnable process. Echocardiographic left ventricular ejection fraction was significantly better in the defer group (60% vs. 57%, p=0.042). In the Super-MIMI (Minimalist Immediate Mechanical Intervention) study,32 there was 1.2% AVC when stenting was delayed to one week, these cases did not receive a glycoprotein IIb/IIIa inhibitor. In the more up-to-date EROSION III (Effective Anti-Thrombotic Therapy Without Stenting: Intravascular Optical Coherence Tomography Based Management in Plaque Erosion) study,59 patients with plaque erosion on OCT had medical treatment in nearly 60% of cases, suggesting that stenting is optional in STEMI. No AVC occurred, despite a 20% rate of glycoprotein IIb/IIIa inhibitor use, compared with a third in the DANAMI-DEFER study, but this study included a direct-acting third-generation P2Y12 inhibitor. STEMI AVC is, therefore, as predictable as in other settings.1,56,57 Importantly, intravascular imaging with OCT has highlighted the diverse aetiologies of STEMI; plaque rupture, plaque erosion, calcium protrusion and a whole host of MINOCA (Myocardial Infarction with Non-Obstructive Coronary Artery) presentations, where stenting is not advisable, such as in spontaneous coronary artery dissection (SCAD) with spreading intramural haematoma, bridging segment, thrombotic or embolic occlusion, epicardial and microvascular vasospasm, etc. STEMI intervention, hence, could be tailored and individualised. The STEMI guidelines written in 2018 will need to be updated.4
Hybrid provisional stenting strategy
Stent use was mostly off-label. The instruction for use (IFU) of DES dictated one stent for a straightforward lesion. In which case, ISR, metal allergy, CTO, STEMI, long lesion requiring two-stent overlap, vein graft, left main, bifurcation, ostial and three-vessel disease were all treated at the operator’s discretion. In spite of this, it drove technical innovation and refinement. The best attended sessions at meetings are case reviews on unforeseen challenges and get-out-of-trouble solutions, apparently worked out on the hoof. The heart is a highly-sensitive life-sustaining organ reacting with immediacy to any invasive procedure. This allows the instant feedback learning process. Any complication arises self-evidently and if not prompt, would be within a short time span. For example, the double-barrel or simultaneous-kissing stenting for bifurcation lesion went out of favour swiftly because of early stent failure.60 Instead, crush stenting remains in use.61 Lim and Dzavik’s crucial description of balloon crush of side-branch stent in 200462 allows for a stepwise approach, giving rise to double-kiss (DK) crush stenting, which is the standard for two-stent strategy.63 In Europe, single-stent technique is favoured and, with DCB, it could be hybrid provisional stenting for complex bifurcation disease as illustrated in figure 2.
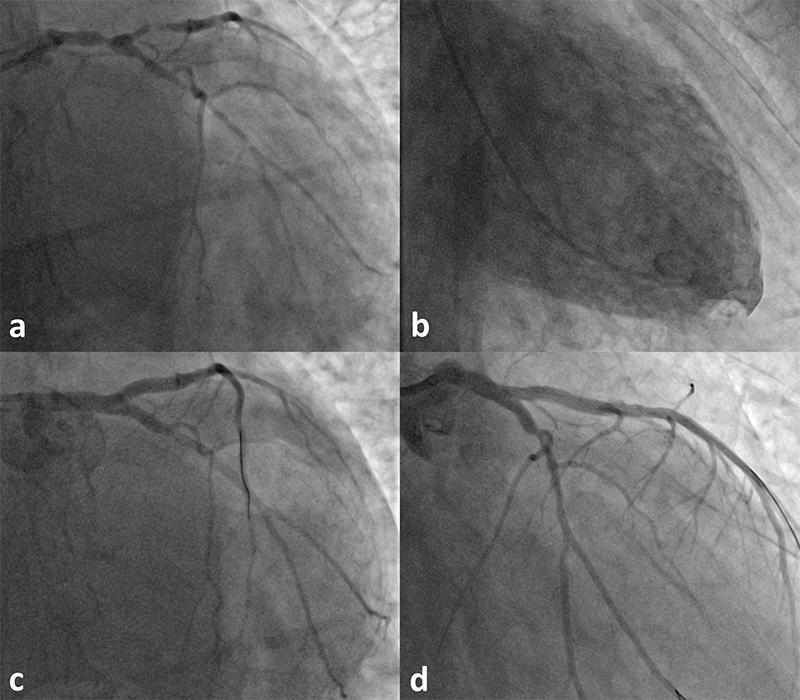
The hybrid provisional stenting strategy limits stenting and allows underfilled and diseased vessel to remodel, as demonstrated by Carlo Di Mario in a CTO case report.64 It is being appraised in bifurcation RCTs.65,66 Any two-stent technique creates stiff-and-thick multi-layered struts, mal-apposition and flow turbulence in the neo-carina, as nidus for stent failure. In contrast, one-stent layer allows conformation to the artery lumen minimising ISR. Further, T-stenting the side branch first guarantees its access after main-branch stent trapping, the sliver of uncovered ostium is then treated with DCB. This technique is quick, it enables easier future re-intervention and it is 5-French compatible, as well as suitable for the unstable patient who tolerates myocardial ischaemia poorly.
Conclusion
It is a good idea to heed many stent manufacturers’ advice to restrict to one non-overlapping DES if stenting is planned, in a straight segment, well-prepared and compliant if calcified, avoiding any bifurcation unless it involves the left main coronary artery. The rest is complemented with DCB, refraining from the spurious or purist argument of using either DES or DCB. Use of DES with DCB at once simplifies and hastens coronary intervention. DCB might be the primary focus for STEMI in the future, as its varied underlying mechanisms are considered. AVC is uncommon with newer antiplatelet drugs, and is both preventable and predictable with practice. DES delays, rather than prevents, target-vessel failure; from POBA that occurs within three to five months to three to five years and continues to fail in due course. With DCB the plaque appears to stabilise with plateauing event rate. Finally, DCB-alone strategy carries no systematic penalty. For the stent enthusiasts, it could be regarded as a defer-stent technique, even if it fails it results in limited stenting in a larger remodelled vessel, but stent deferment tends to be a long-term investment.
Key messages
- Drug-coated balloon reduces coronary restenosis from plain-old-balloon-angioplasty, matching that of drug-eluting stent in de novo small vessel disease
- It has been evaluated in the whole range of coronary artery disease and its use is increasing, especially in the Far East
- It causes luminal enlargement due to positive vascular remodelling, preserves endothelial function, potentially stabilises the plaque and speculatively improves cardiac function. Although its procedural outcome, as with stenting, depends critically on lesion modification, i.e. ‘failure to prepare is preparing to fail’
- Abrupt vessel closure after drug-coated balloon is preventable, predictable and is no more frequent than with stent thrombosis
- When drug-coated balloon is used with drug-eluting stent it limits stenting and simplifies complex coronary artery anatomy
Conflicts of interest
None declared.
Funding
None.
Patient consent
The patients have kindly given their informed consent for their cases to be shared for learned communication.
Acknowledgements
The author trained at the Toronto General Hospital (2003–2005) under Vladimir Dzavik. This started with the severe acute respiratory syndrome (SARS CoV-1) outbreak, albeit at the tail end. Procedures were performed with personal protection equipment as was the case with SARS CoV-2 or COVID-19 in the UK. Over the course of his fellowship, the prevailing philosophy of procedural success there, as was elsewhere, was defined by stenting. Brachytherapy was employed for repeated bare-metal stent ISR and the first-generation DES use started to pick up. Reports of late-acquired stent mal-apposition and thrombosis emerged with post-marketing surveillance after two years, resulting in National Institute for Health and Care Excellence (NICE) guidelines in 2008, which also took into account the costs; restricting second-generation DES to small vessel <3.0 mm in diameter and lesion >15 mm in length. Under this circumstance, the author’s use of DES was <30% and the rest were bare-metal stent and POBA plus thrombectomy in STEMI. The author’s first use of DCB was a DIOR in a 65-year-old man with CKD and right coronary artery ISR on 10 December 2009. It failed within six weeks. The second case was for a de novo ostial obtuse marginal branch lesion in a 59-year-old man with stable angina on 11 January 2010 and he remains well beyond 12 years. The author attended Jim Nolan’s Transradial Masterclass in Manchester on 20–21 November 2014. Simon Eccleshall presented an interesting de novo DCB case in the evening angiographic review session. The author asked him which product he used. Alex Grimster (former head of physiology) put it on the shelves within six weeks. The author participated in Simon Eccleshall’s DCB meeting in Birmingham on 4 September 2015 and the next one on 29 September 2016 sharing his early DCB experience. In the following meeting on 28 September 2017, the author presented his LAD STEMI series and postulated on the stented LAD’s impact on cardiac function. The author thanks the clinical seniors; Stephen Brecker, Rajan Sharma and Manav Sohal for their insightful and progressive leadership; also expresses gratitude to Mary Keal (Matron), Dinesh Sajnani (Head of Radiology), the patients treated by the author including the left main bifurcation case described in this manuscript, Klio Konstantinou and EnHui Yong (trainees) who assisted in the procedure, as illustrated in this report, and all the cardiology staff who selflessly looked after these patients. Finally, this article is the author’s personal viewpoint written from his own experience, interpretation of literature and through interactions with and listening to talks given by experts in the field; Bruno Scheller, Franz Kleber, Klaus Bonaventura, Simon Eccleshall, Sandeep Basavarajaiah, Bernardo Cortese and Tuomas Rissanen.
References
1. Jeger RV, Eccleshall S, Wan Ahmad WA et al. Drug-coated balloons for coronary artery disease: third report of the International DCB Consensus Group. JACC Cardiovasc Interv 2020;13:1391–402. https://doi.org/10.1016/j.jcin.2020.02.043
2. Kleber FX, Schulz A, Waliszewski M et al. Local paclitaxel induces late lumen enlargement in coronary arteries after balloon angioplasty. Clin Res Cardiol 2015;104:217–25. https://doi.org/10.1007/s00392-014-0775-2
3. Scheller B, Speck U, Abramjuk C, Bernhardt U, Bohm M, Nickenig G. Paclitaxel balloon coating, a novel method for prevention and therapy of restenosis. Circulation 2004;110:810–14. https://doi.org/10.1161/01.CIR.0000138929.71660.E0
4. Neumann FJ, Sousa-Uva M, Ahlsson A et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J 2019;40:87–165. https://doi.org/10.1093/eurheartj/ehy855
5. Latib A, Ruparelia N, Menozzi A et al. 3-year follow-up of the Balloon Elution and Late Loss Optimization Study (BELLO). JACC Cardiovasc Interv 2015;8:1132–4. https://doi.org/10.1016/j.jcin.2015.04.008
6. Jeger RV, Farah A, Ohlow MA et al. Drug-coated balloons for small coronary artery disease (BASKET-SMALL 2): an open-label randomised non-inferiority trial. Lancet 2018;392:849–56. https://doi.org/10.1016/S0140-6736(18)31719-7
7. Rissanen TT, Uskela S, Eranen J et al. Drug-coated balloon for treatment of de-novo coronary artery lesions in patients with high bleeding risk (DEBUT): a single-blind, randomised, non-inferiority trial. Lancet 2019;394:230–9. https://doi.org/10.1016/S0140-6736(19)31126-2
8. Chiew KLX, Lim PO. Three-year outcome with drug-coated balloon percutaneous coronary intervention in coronary Takayasu arteritis: a case review. Catheter Cardiovasc Interv 2021;97:841–6. https://doi.org/10.1002/ccd.29099
9. Li QY, Chang MY, Wang XY et al. Efficacy and safety of drug-coated balloon in the treatment of acute myocardial infarction: a meta-analysis of randomized controlled trials. Sci Rep 2022;12:6552. https://doi.org/10.1038/s41598-022-10124-z
10. Vos NS, Fagel ND, Amoroso G et al. Paclitaxel-coated balloon angioplasty versus drug-eluting stent in acute myocardial infarction: the REVELATION randomized trial. JACC Cardiovasc Interv 2019;12:1691–9. https://doi.org/10.1016/j.jcin.2019.04.016
11. Erdogan E, Li Z, Zhu YX et al. DCB combined with provisional DES implantation in the treatment of De Novo Medina 0,1,0 or 0,0,1 left main coronary bifurcation lesions: a proof-of-concept study. Anatol J Cardiol 2022;26:218–25. https://doi.org/10.5152/AnatolJCardiol.2021.1157
12. Gunawardena TD, Corballis N, Merinopoulos I et al. Drug-coated balloon vs. drug-eluting stents for de novo unprotected left main stem disease: the SPARTAN-LMS study. J Cardiovasc Dev Dis 2023;10:84. https://doi.org/10.3390/jcdd10020084
13. Jiang ZM, Liu L. Drug-coated versus uncoated balloon for side branch protection in coronary bifurcation lesions treated with provisional stenting using drug-eluting stents: a meta-analysis. Int J Clin Pract 2022;2022:5892589. https://doi.org/10.1155/2022/5892589
14. Shin ES, Jun EJ, Kim S et al. Clinical impact of drug-coated balloon-based percutaneous coronary intervention in patients with multivessel coronary artery disease. JACC Cardiovasc Interv 2023;16:292–9. https://doi.org/10.1016/j.jcin.2022.10.049
15. Yu X, Wang X, Ji F et al. A non-inferiority, randomized clinical trial comparing paclitaxel-coated balloon versus new-generation drug-eluting stents on angiographic outcomes for coronary de novo lesions. Cardiovasc Drugs Ther 2022;36:655–64. https://doi.org/10.1007/s10557-021-07172-4
16. Aziz A, Bhatia G, Pitt M et al. Intravascular lithotripsy in calcified-coronary lesions: a real-world observational, European multicenter study. Catheter Cardiovasc Interv 2021;98:225–35. https://doi.org/10.1002/ccd.29263
17. Rissanen TT, Uskela S, Siljander A et al. Percutaneous coronary intervention of complex calcified lesions with drug-coated balloon after rotational atherectomy. J Interv Cardiol 2017;30:139–46. https://doi.org/10.1111/joic.12366
18. Shiraishi J, Koshi N, Matsubara Y et al. Stent-less percutaneous coronary intervention using rotational atherectomy and drug-coated balloon: a case series and a mini review. Cardiovasc Revasc Med 2018;19:705–11. https://doi.org/10.1016/j.carrev.2018.02.007
19. Zheng B, Yi T, Wu Q, Bai F, Li J. Drug-coated balloon treatment for possible sequelae of Kawasaki disease evaluated by multi-modalities. Int Heart J 2022;63:773–6. https://doi.org/10.1536/ihj.21-593
20. Volz S, Angeras O, Odenstedt J et al. Sustained risk of stent thrombosis and restenosis in first generation drug-eluting stents after one decade of follow-up: a report from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR). Catheter Cardiovasc Interv 2018;92:E403–E409. https://doi.org/10.1002/ccd.27655
21. Jolly SS, Yusuf S, Cairns J et al. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet 2011;377:1409–20. https://doi.org/10.1016/S0140-6736(11)60404-2
22. Valgimigli M, Frigoli E, Leonardi S et al. Radial versus femoral access and bivalirudin versus unfractionated heparin in invasively managed patients with acute coronary syndrome (MATRIX): final 1-year results of a multicentre, randomised controlled trial. Lancet 2018;392:835–48. https://doi.org/10.1016/S0140-6736(18)31714-8
23. Meier B, Bachmann D, Luscher T. 25 years of coronary angioplasty: almost a fairy tale. Lancet 2003;361:527. https://doi.org/10.1016/S0140-6736(03)12470-1
24. Grüntzig A. Transluminal dilatation of coronary-artery stenosis. Lancet 1978;311:263. https://doi.org/10.1016/S0140-6736(78)90500-7
25. Barton M, Grüntzig J, Husmann M, Rosch J. Balloon angioplasty – the legacy of Andreas Grüntzig, M.D. (1939–1985). Front Cardiovasc Med 2014;1:15. https://doi.org/10.3389/fcvm.2014.00015
26. Anderson HVS. Andreas R. Gruentzig, MD (1939–1985). Cardiology 2022;147:107–12. https://doi.org/10.1159/000519303
27. Meier B. His master’s art, Andreas Grüntzig’s approach to performing and teaching coronary angioplasty. EuroIntervention 2017;13:15–27. https://doi.org/10.4244/EIJV13I1A2
28. Hartzler GO. PTCA in evolution: why is it so popular? Cleve Clin J Med 1990;57:121–4. https://doi.org/10.3949/ccjm.57.2.121
29. Sowtgon E, de Bono D, Gribbin B, Man FS, Silverton P. Coronary angioplasty in the United Kingdom. Report of a Working Party of the British Cardiac Society. Br Heart J 1991;66:325–31. https://doi.org/10.1136/hrt.66.4.325
30. Stone GW, Grines CL, Cox DA et al. Comparison of angioplasty with stenting, with or without abciximab, in acute myocardial infarction. N Engl J Med 2002;346:957–66. https://doi.org/10.1056/NEJMoa013404
31. Kelbaek H, Hofsten DE, Kober L et al. Deferred versus conventional stent implantation in patients with ST-segment elevation myocardial infarction (DANAMI 3-DEFER): an open-label, randomised controlled trial. Lancet 2016;387:2199–206. https://doi.org/10.1016/S0140-6736(16)30072-1
32. Mester P, Bouvaist H, Delarche N et al. At least seven days delayed stenting using minimalist immediate mechanical intervention (MIMI) in ST-segment elevation myocardial infarction: the SUPER-MIMI study. EuroIntervention 2017;13:390–6. https://doi.org/10.4244/EIJ-D-16-00667
33. Adriaenssens T, Joner M, Godschalk TC et al. Optical coherence tomography findings in patients with coronary stent thrombosis: a report of the PRESTIGE Consortium (Prevention of Late Stent Thrombosis by an Interdisciplinary Global European Effort). Circulation 2017;136:1007–21. https://doi.org/10.1161/CIRCULATIONAHA.117.026788
34. Kong MG, Han JK, Kang JH et al. Clinical outcomes of long stenting in the drug-eluting stent era: patient-level pooled analysis from the GRAND-DES registry. EuroIntervention 2021;16:1318–25. https://doi.org/10.4244/EIJ-D-19-00296
35. Kereiakes DJ, Windecker S, Jobe RL et al. Clinical outcomes following implantation of thin-strut, bioabsorbable polymer-coated, everolimus-eluting SYNERGY stents. Circ Cardiovasc Interv 2019;12:e008152. https://doi.org/10.1161/CIRCINTERVENTIONS.119.008152
36. Brophy JM, Belisle P, Joseph L. Evidence for use of coronary stents. A hierarchical bayesian meta-analysis. Ann Intern Med 2003;138:777–86. https://doi.org/10.7326/0003-4819-138-10-200305200-00005
37. Brodie BR, Pokharel Y, Garg A et al. Very late hazard with stenting versus balloon angioplasty for ST-elevation myocardial infarction: a 16-year single-center experience. J Interv Cardiol 2014;27:21–8. https://doi.org/10.1111/joic.12082
38. Lim PO. Angina with coronary microvascular dysfunction and its physiological assessment: a review with cases. Br J Cardiol 2022;29:13. https://doi.org/10.5837/bjc.2022.013
39. Boden WE, Marzilli M, Crea F et al. Evolving management paradigm for stable ischemic heart disease patients: JACC review topic of the week. J Am Coll Cardiol 2023;81:505–14. https://doi.org/10.1016/j.jacc.2022.08.814
40. Al-Lamee R, Thompson D, Dehbi HM et al. Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomised controlled trial. Lancet 2018;391:31–40. https://doi.org/10.1016/S0140-6736(17)32714-9
41. Maron DJ, Hochman JS, Reynolds HR et al. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med 2020;382:1395–407. https://doi.org/10.1056/NEJMoa1915922
42. Herzog CA, Simegn MA, Xu Y et al. Kidney transplant list status and outcomes in the ISCHEMIA-CKD trial. J Am Coll Cardiol 2021;78:348–61. https://doi.org/10.1016/j.jacc.2021.05.001
43. Perera D, Clayton T, O’Kane PD et al. Percutaneous revascularization for ischemic left ventricular dysfunction. N Engl J Med 2022;387:1351–60. https://doi.org/10.1056/NEJMoa2206606
44. Coughlan JJ, Raber L, Brugaletta S et al. Sex differences in 10-year outcomes after percutaneous coronary intervention with drug-eluting stents: insights from the DECADE cooperation. Circulation 2023;147:575–85. https://doi.org/10.1161/CIRCULATIONAHA.122.062049
45. Onuma Y, Chevalier B, Ono M et al. Bioresorbable scaffolds versus everolimus-eluting metallic stents: five-year clinical outcomes of the randomised ABSORB II trial. EuroIntervention 2020;16:e938–e941. https://doi.org/10.4244/EIJ-D-20-00024
46. Venetsanos D, Omerovic E, Sarno G et al. Long term outcome after treatment of de novo coronary artery lesions using three different drug coated balloons. Int J Cardiol 2021;325:30–6. https://doi.org/10.1016/j.ijcard.2020.09.054
47. Lee SY, Cho YK, Kim SW et al. Clinical results of drug-coated balloon treatment in a large-scale multicenter Korean registry study. Korean Circ J 2022;52:444–54. https://doi.org/10.4070/kcj.2021.0261
48. Merinopoulos I, Gunawardena T, Wickramarachchi U et al. Long-term safety of paclitaxel drug-coated balloon-only angioplasty for de novo coronary artery disease: the SPARTAN DCB study. Clin Res Cardiol 2021;110:220–7. https://doi.org/10.1007/s00392-020-01734-6
49. Scheller B, Vukadinovic D, Jeger R et al. Survival after coronary revascularization with paclitaxel-coated balloons. J Am Coll Cardiol 2020;75:1017–28. https://doi.org/10.1016/j.jacc.2019.11.065
50. Kawai T, Watanabe T, Yamada T et al. Coronary vasomotion after treatment with drug-coated balloons or drug-eluting stents: a prospective, open-label, single-centre randomised trial. EuroIntervention 2022;18:e140–e148. https://doi.org/10.4244/EIJ-D-21-00636
51. Kim S, Lee JS, Kim YH et al. Favorable vasomotor function after drug-coated balloon-only angioplasty of de novo native coronary artery lesions. J Clin Med 2022;11:299. https://doi.org/10.3390/jcm11020299
52. Yamamoto T, Sawada T, Uzu K, Takaya T, Kawai H, Yasaka Y. Possible mechanism of late lumen enlargement after treatment for de novo coronary lesions with drug-coated balloon. Int J Cardiol 2020;321:30–7. https://doi.org/10.1016/j.ijcard.2020.07.028
53. Chowdhury MM, Singh K, Albaghdadi MS et al. Paclitaxel drug-coated balloon angioplasty suppresses progression and inflammation of experimental atherosclerosis in rabbits. JACC Basic Transl Sci 2020;5:685–95. https://doi.org/10.1016/j.jacbts.2020.04.007
54. Rothberg MB, Celestin C, Fiore LD, Lawler E, Cook JR. Warfarin plus aspirin after myocardial infarction or the acute coronary syndrome: meta-analysis with estimates of risk and benefit. Ann Intern Med 2005;143:241–50. https://doi.org/10.7326/0003-4819-143-4-200508160-00005
55. Huber MS, Mooney JF, Madison J, Mooney MR. Use of a morphologic classification to predict clinical outcome after dissection from coronary angioplasty. Am J Cardiol 1991;68:467–71. https://doi.org/10.1016/0002-9149(91)90780-O
56. Merinopoulos I, Wickramarachchi U, Wardley J et al. Day case discharge of patients treated with drug coated balloon only angioplasty for de novo coronary artery disease: a single center experience. Catheter Cardiovasc Interv 2020;95:105–08. https://doi.org/10.1002/ccd.28217
57. Shin ES, Ann SH, Balbir Singh G, Lim KH, Kleber FX, Koo BK. Fractional flow reserve-guided paclitaxel-coated balloon treatment for de novo coronary lesions. Catheter Cardiovasc Interv 2016;88:193–200. https://doi.org/10.1002/ccd.26257
58. Jinnouchi H, Sakakura K, Yanase T et al. Impact of stent edge dissection detected by optical coherence tomography after current-generation drug-eluting stent implantation. PLoS One 2021;16:e0259693. https://doi.org/10.1371/journal.pone.0259693
59. Jia H, Dai J, He L et al. EROSION III: a multicenter RCT of OCT-guided reperfusion in STEMI with early infarct artery patency. JACC Cardiovasc Interv 2022;15:846–56. https://doi.org/10.1016/j.jcin.2022.01.298
60. Morris PD, Iqbal J, Chiastra C, Wu W, Migliavacca F, Gunn JP. Simultaneous kissing stents to treat unprotected left main stem coronary artery bifurcation disease; stent expansion, vessel injury, hemodynamics, tissue healing, restenosis, and repeat revascularization. Catheter Cardiovasc Interv 2018;92:E381–E392. https://doi.org/10.1002/ccd.27640
61. Raphael CE, O’Kane PD, Johnson TW et al. Evolution of the crush technique for bifurcation stenting. JACC Cardiovasc Interv 2021;14:2315–26. https://doi.org/10.1016/j.jcin.2021.08.048
62. Lim PO, Dzavik V. Balloon crush: treatment of bifurcation lesions using the crush stenting technique as adapted for transradial approach of percutaneous coronary intervention. Catheter Cardiovasc Interv 2004;63:412–16. https://doi.org/10.1002/ccd.20179
63. Chen X, Li X, Zhang JJ et al. 3-year outcomes of the DKCRUSH-V trial comparing DK crush with provisional stenting for left main bifurcation lesions. JACC Cardiovasc Interv 2019;12:1927–37. https://doi.org/10.1016/j.jcin.2019.04.056
64. Ciardetti N, Nardi G, Di Mario C. Direct high-frequency intravascular ultrasound visualization of drug delivery after paclitaxel urea eluting balloon angioplasty. Eur Heart J 2023;44:1096. https://doi.org/10.1093/eurheartj/ehad020
65. Gao XF, Ge Z, Kan J et al. Rationale and design for comparison of non-compliant balloon with drug-coating balloon angioplasty for side branch after provisional stenting for patients with true coronary bifurcation lesions: a prospective, multicentre and randomised DCB-BIF trial. BMJ Open 2022;12:e052788. https://doi.org/10.1136/bmjopen-2021-052788
66. Guo Q, Peng L, Rao L et al. The “L-Sandwich” strategy for true coronary bifurcation lesions: a randomized clinical trial. J Interv Cardiol 2023;2023:6889836. https://doi.org/10.1155/2023/6889836
