Atrial fibrillation (AF) is responsible for significant patient morbidity, and obesity is a major contributor to AF incidence and symptom burden. Weight loss has been shown to positively modify AF symptoms, but weight loss in a real-world population is often only temporary. This randomised study set out to examine if smartphone-based app technology could increase weight loss in a patient population with obesity and AF.
Individuals were screened following outpatient referral to Leeds Teaching Hospitals NHS Trust for symptomatic AF. Block randomisation was performed to allocate the treatment groups to either clinical follow-up or app-based follow-up, with weight loss updates planned fortnightly. Patients randomised to clinical follow-up received nurse-led telephone calls every two weeks, while those in the app arm received automated reminder messages every two weeks. Final follow-up at six months included clinical and weight data and a quality-of-life questionnaire.
Sixty-four patients underwent randomisation. No significant difference in weight loss was seen between the groups. Patient engagement was far more consistent in the telephone follow-up group.
In conclusion, no significant difference in weight loss was seen between the two groups, despite patient education on the value of weight loss to improve their AF symptoms.
Introduction
Atrial fibrillation (AF) is a common arrhythmia responsible for significant patient morbidity, including stroke, heart failure and intrusive palpitations.1 Obesity is well recognised as a contributor to AF incidence and symptom burden.2 Weight loss has been shown to reduce occurrence of AF and increase the likelihood of maintaining sinus rhythm after cardioversion or catheter ablation for AF.3–5
Although it has been demonstrated that weight loss can reduce AF burden in a trial setting, it is well recognised that motivation for sustained weight loss is low in a real-world population, and often any weight loss achieved is temporary.6 It is, therefore, vital that effective strategies are utilised to ensure patients are motivated and educated to achieve sustained weight loss; to not only improve symptoms of AF, but also to reduce hospital admissions and the need for invasive procedures, such as AF ablation.
App-based digital health solutions are increasingly popular and, with the ubiquity of smartphones, offer an important potential avenue for patients to receive ongoing motivation and education to sustain weight loss. AVATR is a smartphone-based app that enables a person to create a digital representation of themselves, which they own and control. The app collects raw health/lifestyle data from smartphones and wearable medical devices, and can share this with medical professionals to receive personalised healthcare. The app does not require large data sets or inferencing from population data, and so is well positioned to support population groups at risk of exclusion and machine bias, such as women, older people and the economically disadvantaged.
Our study set out to examine the efficacy of this smartphone-based technology to educate patients around AF and weight loss, and by providing continued support, ensure the patient remained focused on achieving sustained weight loss.
Method
Individuals were screened for enrolment following outpatient referral to Leeds Teaching Hospitals NHS Trust for symptomatic AF. Inclusion criteria included: age ≥18 years; body mass index (BMI) >27 kg/m2; symptomatic AF; commitment to use the AVATR app and attend follow-up appointments. Exclusion criteria included: life-expectancy <1 year; inability to commit to follow-up; prior recruitment to ongoing weight-loss studies; lack of smartphone app compatibility; and not providing expressed informed consent to using the app.
Each participant’s understanding of the impact of weight loss on AF symptoms was assessed. Guidance was provided to the participant advising that for every increase of one unit in BMI there will be an increased risk of AF by 5%, while a reduction of one unit could provide up to 10% reduction in symptoms.7 Motivation and confidence to change was assessed by a subjective scoring of 0 to 10, 0 being not motivated and 10 being highly motivated. Any score less than seven was discussed to identify the cause of the lower score. Quality of life was assessed using the EQ-5D-5L questionnaire. The participant chose their favoured weight-loss method from several different programmes, and were asked to set a weight-loss goal.
Block randomisation with sealed envelopes was performed to allocate the treatment groups to either clinical telephone follow-up or AVATR follow-up. Envelopes were opened at the time of enrolment and the randomisation sequence was concealed from both clinical staff and patients. Patients randomised to clinical follow-up received nurse-led telephone calls every two weeks, while those in the AVATR arm received automated reminder messages every two weeks to remind participants to record weight and activity within the app, with any clinical issues communicated via the app. Final follow-up at six months included clinical and weight data and a quality-of-life questionnaire.
The primary outcome was weight loss at six month follow-up compared with baseline. Secondary outcomes included improvement in quality of life and reduction of symptoms (EQ-5D-5L), patient satisfaction, time saved by virtual follow-up and compliance.
Results
Table 1. Baseline characteristics
| Characteristic | AVATR follow-up (n=22) |
Phone follow-up (n=32) |
| Mean age ± SD, years | 63 ± 7.7 | 60 ± 9.5 |
| Female participants, n (%) | 11 (50) | 15 (47) |
| Mean BMI ± SD, kg/m2 | 38.3 ± 4.6 | 37.9 ± 6.6 |
| Sinus rhythm at baseline, n (%) | 4 (18) | 9 (28) |
| Mean SBP ± SD, mmHg | 133.1 ± 13.4 | 127.3 ± 16.7 |
| Mean DBP ± SD, mmHg | 81.3 ± 9.5 | 77.5 ± 13.5 |
| Mean heart rate ± SD, bpm | 71.9 ± 14.3 | 77.4 ± 14.1 |
| Hypertension, n (%) | 14 (64) | 12 (38) |
| Diabetes, n (%) | 5 (23) | 6 (19) |
| Ischaemic heart disease, n (%) | 2 (9) | 1 (3) |
| Congestive heart failure, n (%) | 4 (18) | 7 (22) |
| Key: BMI = body mass index; DBP = diastolic blood pressure; SBP = systolic blood pressure; SD = standard deviation | ||
A total of 148 eligible referrals were received, 84 patients declined to consent and 64 underwent randomisation. Thirty-three were randomised to telephone call, 28 were randomised to AVATR, and three patients could not be contacted. Six patients from the AVATR arm and one patient from the telephone follow-up arm withdrew from the study prior to the initial visit. Baseline characteristics are presented in table 1.
Median weight loss was 3.95 kg (median loss of 1.6% initial bodyweight) in the phone follow-up arm versus 2.25 kg (median loss of 3.6% initial bodyweight) in the app follow-up arm. No significant difference in weight loss was seen when assessed on an intention-to-treat basis using Mann-Whitney U test (figure 1).
Patient engagement was far more consistent in the telephone follow-up group, with a final weight available at the end of the 26-week follow-up period from 31 out of the 32 patients (97%) in that arm, and a median 13 contacts out of a possible 13. Conversely, as indicated in figure 1B, just five of the 22 patients (23%) in the app follow-up arm had a weight measurement in the final week, with a median of 2.5 contacts per patient out of 13. Completion of final follow-up questionnaires was insufficient in the AVATR app group to determine any meaningful outcomes.
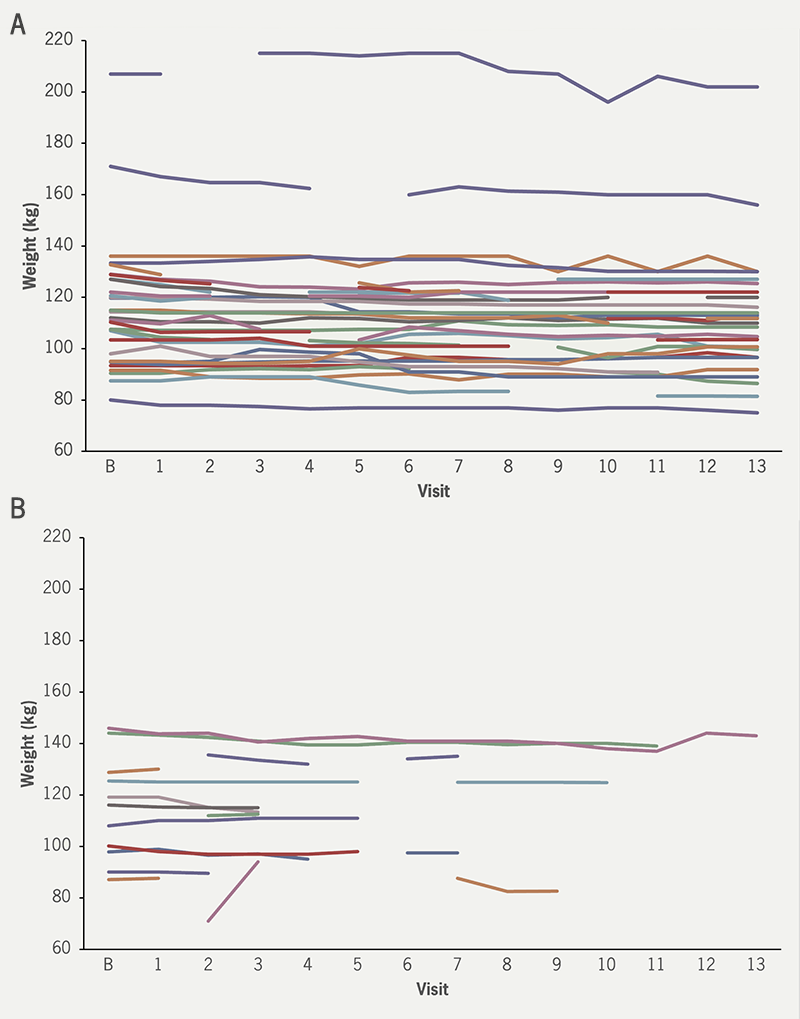
| Individual patients are represented by individual lines. No significant differences in weight loss were seen. Key: B = baseline |
Discussion
This study of weight loss guided by telephone follow-up or smartphone-based app technology demonstrated no significant difference in weight loss between the two groups. A high proportion of eligible patients declined to take part in the study at all, potentially reflecting initial motivation in this patient group as a whole.
Ongoing motivation was assessed on a pragmatic basis by whether the patient engaged with follow-up visits, rather than using specific motivation assessment tools. This may be considered a limitation of the study. Non-compliance with the diet, as well as an inability to exercise were common reasons for patients to report why they did not lose weight.
Patient engagement with the app was poor, with limited available follow-up data and few patient contacts, despite regular automated reminders. Additional features to support patient engagement within the app were not used in this trial, and due to the COVID pandemic there was no face-to-face training in how to use the app. Usability of the AVATR app may, therefore, have limited data collection and reduced compliance, but the interface of the app itself cannot be commented on, as feedback for this was not collected.
The perceived social pressure of a healthcare professional personally collecting and monitoring data may have been a motivating factor for those patients in the phone follow-up arm to remain engaged compared with automated reminders. This level of clinical input to aid weight-loss motivation exceeds what is possible for ‘real-world’ patients, and was intended to mirror the app capabilities. There was a numerically higher number of patients in sinus rhythm at baseline assessment in this arm, which may lead to improved exercise capacity. However, this baseline difference and continued patient engagement was still not sufficient to lead to sustained, significant weight loss in this group.
Although this study did not show improved weight-loss outcomes with app-based technology, the use of information technology and apps in healthcare is a field that is continually growing and developing. It may still have a pivotal role, in conjunction with healthcare professional input, to encourage weight loss in the future. However, the most frequent users of digital health technologies are often those with least need for them, and technological improvements alone are unlikely to solve the problem of how to connect with those patients who will benefit most, and inspire sustainable change.
Key messages
- Obesity is a risk factor for atrial fibrillation, and weight loss improves symptoms
- Digital health technologies are increasingly popular, and may help improve weight-loss motivation
- A smartphone app designed to improve patient engagement and motivation did not affect weight loss in our randomised study
- Patient engagement was poor with the app, and weight-loss motivation remains difficult to achieve
Conflicts of interest
MHT has received fellowship funding and proctoring fees from Biosense Webster and research grants from AliveCor. MW is CEO and founder of Inavya Ventures Ltd. TAS, EM, LL: none declared.
Funding
This research was supported by Inavya Ventures Ltd., who provided the app and hardware. Additional funding was provided by the Leeds Electrophysiology Research fund. The study was adopted by the NIHR clinical research network portfolio.
Study approval
Ethical approval was provided by North of Scotland Research Ethics Committee – reference 20/NS/0045. Written informed consent was obtained from all participants in concordance with the Declaration of Helsinki.
References
1. Lopez-Jimenez F, Almahmeed W, Bays H et al. Obesity and cardiovascular disease: mechanistic insights and management strategies. A joint position paper by the World Heart Federation and World Obesity Federation. Eur J Prev Cardiol 2022;29:2218–37. https://doi.org/10.1093/eurjpc/zwac187
2. Vyas V, Lambiase P. Obesity and atrial fibrillation: epidemiology, pathophysiology and novel therapeutic opportunities. Arrhythmia Electrophysiol Rev 2019;8:28–36. https://doi.org/10.15420/aer.2018.76.2
3. Abed HS, Wittert GA, Leong DP et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA 2013;310:2050–60. https://doi.org/10.1001/jama.2013.280521
4. Middeldorp ME, Pathak RK, Meredith M et al. PREVEntion and regReSsive Effect of weight-loss and risk factor modification on Atrial Fibrillation: the REVERSE-AF study. Europace 2018;20:1929–35. https://doi.org/10.1093/europace/euy117
5. Pathak RK, Middeldorp ME, Meredith M et al. Long-term effect of goal-directed weight management in an atrial fibrillation cohort: a long-term follow-up study (LEGACY). J Am Coll Cardiol 2015;65:2159–69. https://doi.org/10.1016/j.jacc.2015.03.002
6. DerSarkissian M, Bhak RH, Huang J et al. Maintenance of weight loss or stability in subjects with obesity: a retrospective longitudinal analysis of a real-world population. Curr Med Res Opin 2017;33:1105–10. https://doi.org/10.1080/03007995.2017.1307173
7. Gami AS, Hodge DO, Herges RM et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol 2007;49:565–71. https://doi.org/10.1016/j.jacc.2006.08.060
