Pathogenic genetic variants in the HCN4 (hyperpolarisation-activated and cyclic nucleotide-gated channel) gene can cause sinus node dysfunction, disrupting the function of the pacemaker region of the heart. Patients with such variants can present with a wide spectrum of arrhythmias but sudden death is generally considered rare. We report two cases from the same family with different outcomes to highlight a potential high-risk phenotype in HCN4-related disease.
Introduction
Hyperpolarisation-activated and cyclic nucleotide-gated (HCN) cation (Na+/K+) currents are generated by four members of the HCN-channel family – HCN1–4.1 The currents of these channels, often designated as If (funny channel) or Ih (hyperpolarisation-activated cation channels), are activated by membrane hyperpolarisation to produce a graded depolarisation in phase 4 of the cardiac action potential. The most prominent channel in the pacemaker region of the heart, essential to the automaticity of the sinus node, is the HCN4 channel.1 Historically, pathogenic variants in HCN4 have been associated with a sick sinus syndrome phenotype.2–4 However, more recent reports have expanded the range of documented arrhythmia, including early-onset atrial fibrillation,5 atrioventricular block,6 and inappropriate sinus tachycardia.7 Aside from arrhythmias, other reports also suggest disruption of HCN4 might affect the structure of the heart and vasculature leading to excessive left ventricular trabeculation4 and/or aortic dilatation;8 common non-coding variants in this gene may increase the incidence of mood disorders, particularly anxiety.9
 |
 |
Case 1
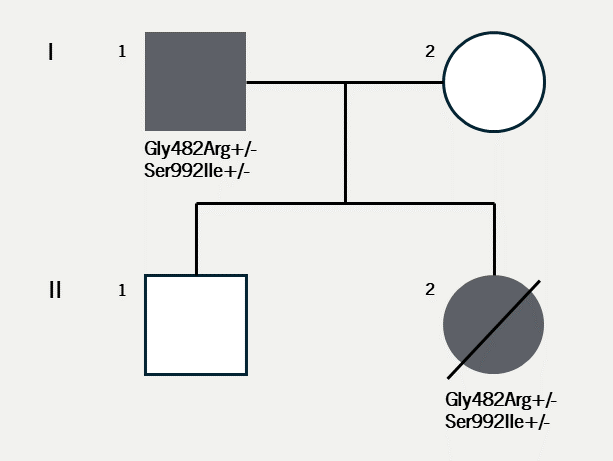
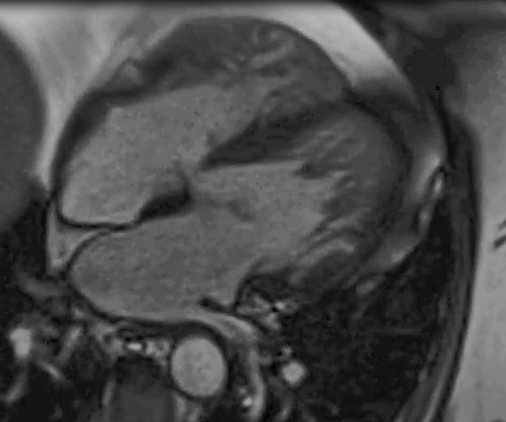
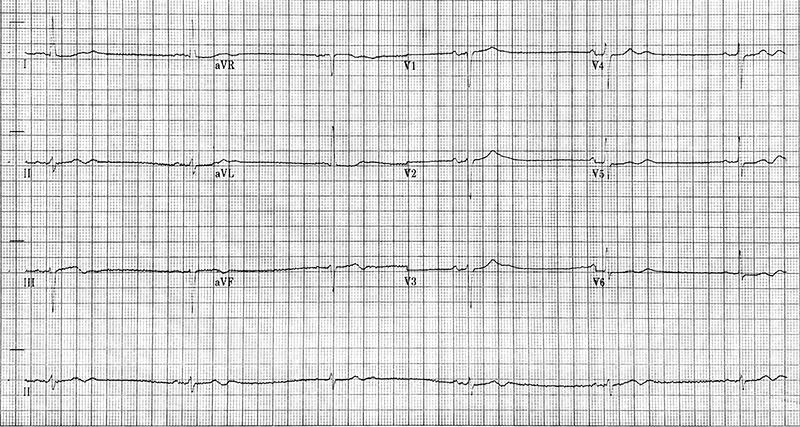
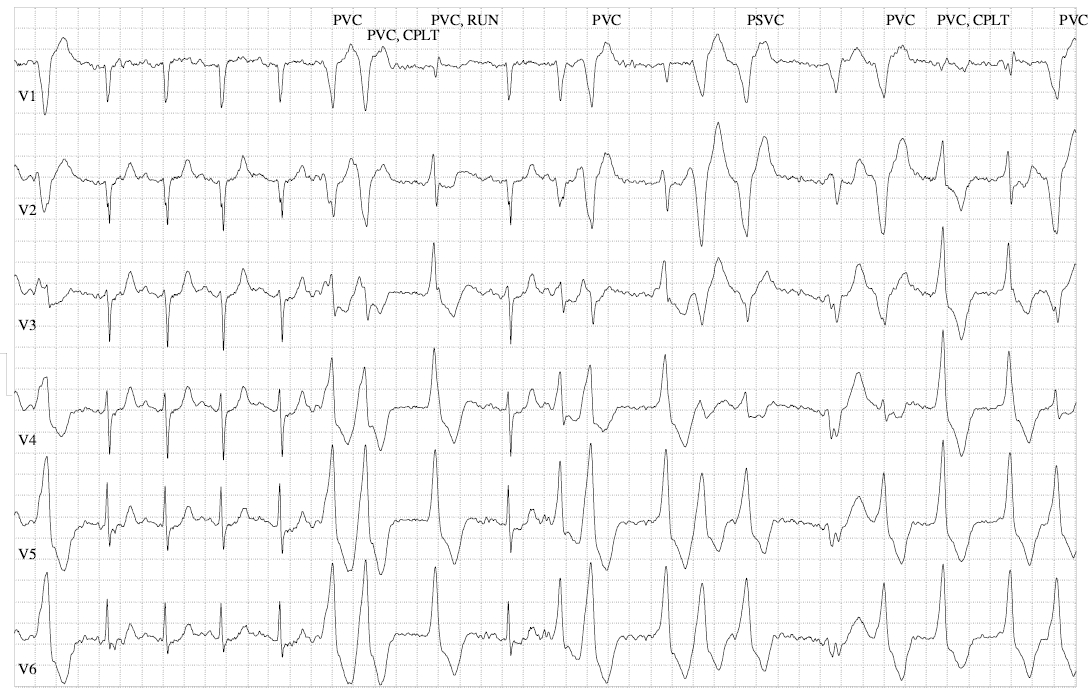
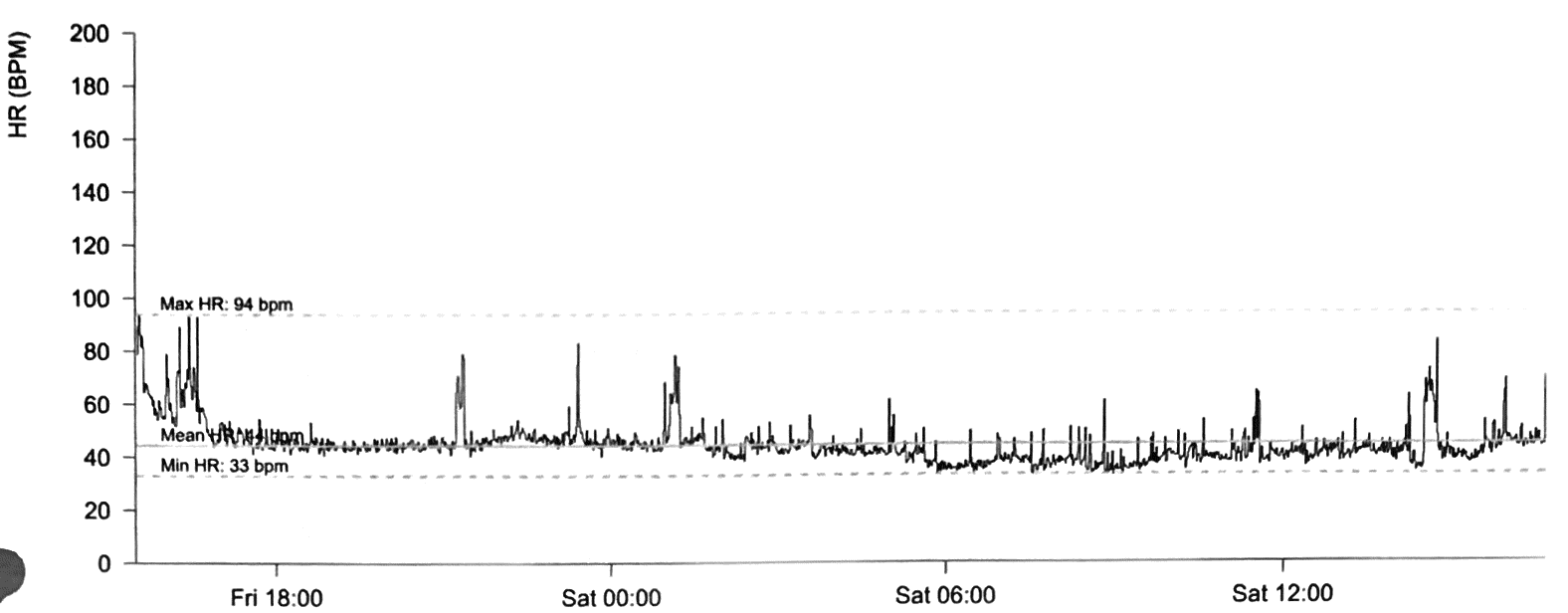
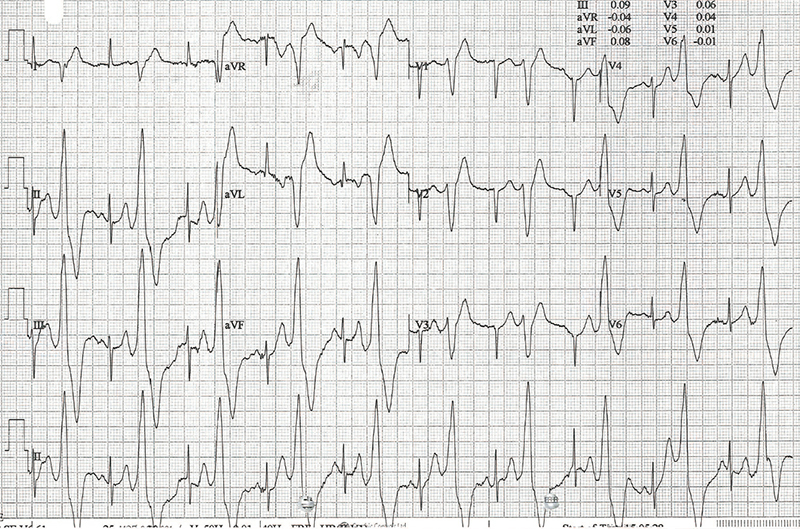
Case 1 (I.1. in figure 1) was diagnosed with asymptomatic sinus bradycardia at 14 years of age and was managed conservatively. In his 50s, he reported breathlessness and palpitations but did not have syncope. He was obese (body mass index [BMI] was 32 kg/m2) and had hypertension, gout, vitamin D deficiency and sleep apnoea. Medical therapy included ramipril 10 mg daily, amlodipine 5 mg daily and vitamin D supplements. A cardiac magnetic resonance imaging scan at 52 years of age showed left ventricular (LV) hypertrabeculation with abnormal LV geometry but normal biventricular function (figure 2 and videos available upon request). He had a sinus bradycardia detected on his resting electrocardiogram (ECG) (figure 3A) and during ambulatory monitoring (rate histogram in figure 3B). Exercise testing at the age of 54 years was discontinued in stage 2 due to the development of frequent ventricular ectopics (VEs), non-sustained ventricular tachycardia (NSVT) and T-wave inversion (TWI) (figure 3C). These consisted of multiple morphologies (both right and left bundle branch blocks [RBBB and LBBB], superior and inferior axes). Ectopy and NSVT originated early in exercise and persisted into the recovery phase. The heart rate (HR) and blood pressure (BP) rose appropriately (HR from 63 beats per minute [bpm] to 114 bpm, BP from 158/98 to 180/98 mmHg). Subsequent 48-hour ambulatory ECG monitoring showed a sinus bradycardia (mean HR 48 bpm) with only a small number of VEs, TWI and no other significant arrhythmias (figure 3D). Case 1 had mild symptoms due to sinus bradycardia and palpitations which correlate with paroxysmal AF.
Case 2
Case 2 (II.2. in figure 1) is the daughter of case 1. She was diagnosed with sinus bradycardia at 11 years of age. In view of her family history, she was referred for genetic testing. This revealed a pathogenic gene change in HCN4 (c.1444G>A, p.(Gly482Arg)) which was assessed as a class 5 variant according to the American College of Medical Genetics Association for Molecular Pathology (ACMG/AMP) joint consensus statement classification.10 This variant has been reported in association with sinus node disease, with and without evidence of cardiomyopathy.4,11,12 It has been reported to present in foetal life in two cases,13 including one case (in this case a different genetic variant, c.1444G>C, causing the same change to the amino acid sequence of the protein) with a de novo genetic presentation. The variant has been shown to segregate with disease in multiple families and it has not been reported in population databases. It causes the substitution of a non-polar amino acid to a positively charged polar amino acid within a functionally important GYG motif in the channel pore domain.14
A second HCN4 variant has also been reported (c.2975G>T, p.(Ser992Ile)). It has been found at a very low frequency in population databases. This was classified as a variant of uncertain significance (VUS), class 3 by ACMG classification.10 Case 1 was subsequently confirmed to be a carrier of both variants, thereby establishing that they are carried in cis (i.e., affecting the same copy of HCN4).
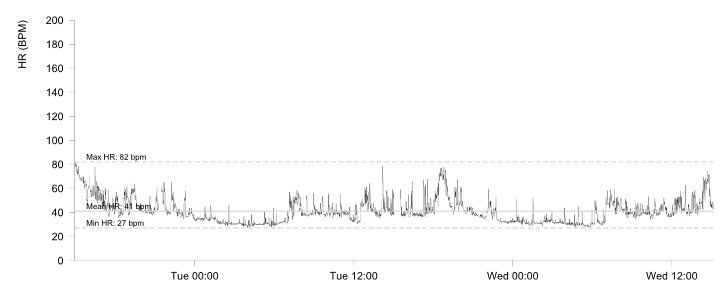
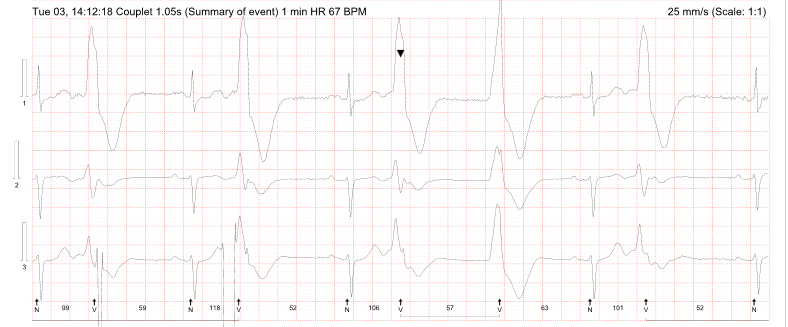
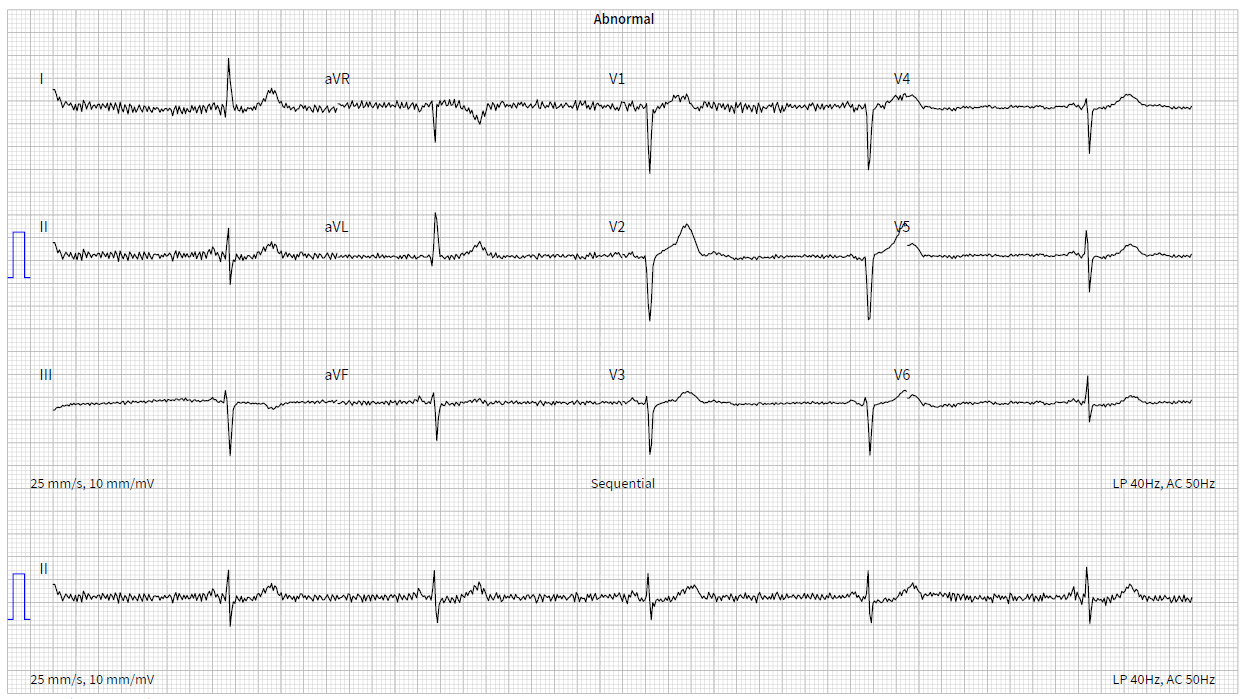
Ambulatory monitoring at age 17 in case 2 showed a mean HR of 40 bpm with frequent VEs (rate histogram figure 3F). Exercise testing showed frequent VEs (left bundle branch block [LBBB] morphology, inferior axis) in early exercise (figure 3G) which persisted into stage 3 but did not become more complex. There were similarities between the ECGs of both cases, with both having prominent U waves, frequent VEs on exercise testing and left-axis deviation. Some of these features, such as the prominent U wave and VEs, have been previously reported in this variant.11 She had good chronotropic response. She was asymptomatic, denying palpitations and syncope. Echocardiography showed mild pulmonary valvular stenosis and mild left pulmonary artery stenosis but normal ventricular morphology and function with no non-compaction. It is unclear whether these structural cardiovascular abnormalities are related to the HCN4 variant. Case 2 was treated with sertraline for anxiety from the age of 19. A repeat exercise test at age 19 was abandoned due to pre-exercise hypertension (180/120 mmHg), felt to be due to anxiety. At age 20, case 2 suffered a road traffic accident. She denied losing consciousness and stated that she had swerved to avoid an oncoming vehicle, hitting the kerb. She did not suffer any injury. The ECG at admission showed a sinus bradycardia of 32 bpm with borderline left-axis deviation, a normal PR interval and a normal corrected-QT interval (QT 510 ms, QTc by the Bazzett formula was 370 ms, figure 3E). However, due to the poor-quality tracing it is possible this was artefactual. One-week later, she was found dead in bed at the age of 20. Toxicology showed pharmacological levels of her usual medication sertraline along with low levels of alprazolam, cannabinoids and alcohol (31 mL/dL). These were not considered likely to have caused her death but, in combination with her heart disease, it is possible they may have been contributory. The cause of death determined by the coroner was acute sinus node dysfunction, but tachyarrhythmia could not be excluded.
Discussion
Both cases were carriers of the same HCN4 haplotype and presented with a similar electrical phenotype, although imaging findings were different with evidence of non-compaction cardiomyopathy in case 1. As both cases had remained largely asymptomatic with conservative management and no worrying events such as syncope, they were deemed as relatively low risk. Furthermore, most of the literature suggests that pathogenic HCN4 variants are not associated with a high risk of sudden cardiac death in asymptomatic individuals. A single instance of unheralded cardiac arrest was found upon literature review in an early report of three families with HCN4-related disease.3 The patient survived the arrest and there was no arrhythmia on subsequent exercise testing, although atrial fibrillation was inducible upon electrophysiological study. A further report described a patient with recurrent syncope who was documented to have polymorphic VT.8,15 This arrhythmia is commonly caused by QT prolongation and in this case was likely to have been triggered by extreme bradycardia, rather than being a primary tachyarrhythmia. Finally, where prophylactic implantable cardiac defibrillators (ICDs) have been fitted, appropriate therapies for tachyarrhythmia are rare.4 Based on these factors, case 2 was evaluated as having insufficient risk of dangerous ventricular arrhythmia to outweigh the risks of ICD therapy.
The molecular genetics of this family is complex; both cases carry two HCN4 missense variants in cis; one pathogenic, the other of uncertain pathogenicity and clinical significance. It is likely that the VUS alone would not cause significant arrhythmias. However, it is possible that it may be acting as a modifier of the phenotype and risk associated with the pathogenic variant. Equally, it is possible that this variant has no significant effect on channel function as it is known that the Gly482Arg substitution is sufficient on its own to cause disease. Variable disease expression is common in inherited heart disease and the phenotypic variation seen in this family may not be explained simply by the HCN4 haplotype. Case 1 has since received a prophylactic transvenous ICD. He has had inappropriate therapies for paroxysmal atrial fibrillation, but no sustained ventricular arrhythmia has been recorded to date. We have recommended genetic cascade screening in relatives, regardless of clinical status.
Conclusion
Tachyarrhythmia and sudden death appear to be rare in HCN4-related disease. Patients without a history of syncope and with good chronotropic response to exercise are reported to have a low risk of events with conservative management and without pacing. There is no consensus over when device therapy is indicated, beyond standard guidelines for pacing, and ICD therapy seems to be rarely necessary. Despite this, arrhythmia in HCN4-related disease is clearly potentially life-threatening and further study is needed to determine risk markers for sudden death in asymptomatic patients. The two unusual and potentially relevant factors in this family were the presence of an additional VUS and the frequency of ectopy, at rest and provoked by exercise. Our intention in reporting this family is to highlight the possibility of a higher-risk subset of patients with HCN4-related disease.
Key messages
- Pathogenic genetic variants in the HCN4 gene are usually associated with sick sinus syndrome; however, sudden death is generally considered rare
- Recent reports have expanded the range of associated arrhythmias, including atrial fibrillation, atrioventricular block and inappropriate sinus tachycardia, and suggest that disruption of HCN4 might affect the structure of the heart (particularly causing excessive left ventricular trabeculation) and vasculature
- Common non-coding variants in the HCN4 gene may increase the incidence of mood disorders, particularly anxiety
- These cases highlight that carriers of disease-causing HCN4 variants should be managed in a specialist setting, with careful evaluation to determine whether they might be at increased risk of sudden cardiac death
- Certain patients may warrant additional precautions, particularly ICD therapy, though evidence is currently lacking in this regard.
Acknowledgement
Images were sourced by Dr Ormerod from clinical data.
Conflicts of interest
None declared.
Funding
None.
Statement of consent
Written informed consent was obtained from case 1 for the publication of their medical case details, including any associated images, diagnostic findings, and clinical history. Case 1 understood that their case details would be made available to the public through the publication of this case report, with measures taken to protect their privacy and anonymity. Case 1 provided consent freely, with the option to withdraw consent at any time before publication, without affecting their medical care or rights. Case 1, being the next of kin of case 2, consented for the inclusion of case 2 in this report.
References
1. Altomare C, Terragni B, Brioschi C et al. Heteromeric HCN1–HCN4 channels: a comparison with native pacemaker channels from the rabbit sinoatrial node. J Physiol 2003;549:347–59. https://doi.org/10.1113/jphysiol.2002.027698
2. Nof E, Luria D, Brass D et al. Point mutation in the HCN4 cardiac ion channel pore affecting synthesis, trafficking, and functional expression is associated with familial asymptomatic sinus bradycardia. Circulation 2007;116:463–70. https://doi.org/10.1161/CIRCULATIONAHA.107.706887
3. Laish-Farkash A, Glikson M, Brass et al. A novel mutation in the HCN4 gene causes symptomatic sinus bradycardia in Moroccan Jews. J Cardiovasc Electrophysiol 2010;21:1365–72. https://doi.org/10.1111/j.1540-8167.2010.01844.x
4. Milano A, Vermeer AMC, Lodder EM et al. HCN4 mutations in multiple families with bradycardia and left ventricular noncompaction cardiomyopathy. J Am Coll Cardiol 2014;64:745–56. https://doi.org/10.1016/j.jacc.2014.05.045
5. Macri V, Mahida SN, Zhang ML et al. A novel trafficking-defective HCN4 mutation is associated with early-onset atrial fibrillation. Heart Rhythm 2014;11:1055–62. https://doi.org/10.1016/j.hrthm.2014.03.002
6. Zhou J, Ding W-G, Makiyama T et al. A novel HCN4 mutation, G1097W, is associated with atrioventricular block. Circulation J 2014;78:938–42. https://doi.org/10.1253/circj.cj-13-0996
7. Baruscotti M, Bucchi A, Milanesi R et al. A gain-of-function mutation in the cardiac pacemaker HCN4 channel increasing cAMP sensitivity is associated with familial Inappropriate sinus tachycardia. Eur Heart J 2017;38:280–8. https://doi.org/10.1093/eurheartj/ehv582
8. Vermeer AMC, Lodder EM, Thomas D et al. Dilation of the aorta ascendens forms part of the clinical spectrum of HCN4 mutations. J Am Coll Cardiol 2016;67:2313–5. https://doi.org/10.1016/j.jacc.2016.01.086
9. Kelmendi B, Holsbach-Beltrame M, McIntosh AM et al. Association of polymorphisms in HCN4 with mood disorders and obsessive compulsive disorder. Neurosci Lett 2011;496:195–9. https://doi.org/10.1016/j.neulet.2011.04.026
10. Richards S, Aziz N, Bale S et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405–24. https://doi.org/10.1038/gim.2015.30
11. Schweizer PA, Schröter J, Greiner S et al. The symptom complex of familial sinus node dysfunction and myocardial noncompaction is associated with mutations in the HCN4 channel. J Am Coll Cardiol 2014;64:757–67. https://doi.org/10.1016/j.jacc.2014.06.1155
12. Millat G, Janin A, de Tauriac O et al. HCN4 mutation as a molecular explanation on patients with bradycardia and non-compaction cardiomyopathy. Eur J Med Genet 2015;58:439–42. https://doi.org/10.1016/j.ejmg.2015.06.004
13. Wacker-Gussmann A, Oberhoffer-Fritz R, Westphal DS et al. The missense variant p.(Gly482Arg) in HCN4 is responsible for fetal tachy-bradycardia syndrome. Heart Rhythm Case Rep 2020;6:352–6. https://doi.org/10.1016/j.hrcr.2020.03.003
14. Ishikawa T, Ohno S, Murakami T et al. Sick sinus syndrome with HCN4 mutations shows early onset and frequent association with atrial fibrillation and left ventricular noncompaction. Heart Rhythm 2017;14:717–24. https://doi.org/10.1016/j.hrthm.2017.01.020
15. Ueda K, Hirano Y, Higashiuesato Y et al. Role of HCN4 channel in preventing ventricular arrhythmia. J Hum Genet 2009;54:115–21. https://doi.org/10.1038/jhg.2008.16
