A 74-year-old man with a heterotopic heart transplant experienced alternating episodes of sustained native heart ventricular tachycardia and prolonged asystole. These were managed with cardioversion, drug therapy and pacemaker insertion. The unique physiology in such patients lends itself to numerous clinical considerations that would otherwise be routine management for most.
Introduction
Patients with end-stage heart failure can be managed with a left ventricular assist device (LVAD) or an orthotropic heart transplant. Historically, heterotopic heart transplants (HHT) were also utilised due to theoretical advantages of assisting the circulation during rejection and in the management of pulmonary hypertension. In an orthotropic heart transplant, the native heart is replaced by the donor, while in a HHT, the donor heart is anastomosed to the native heart, such that both function in tandem.1 Typically, the donor aorta is attached to the recipient’s aorta and the donor right ventricle is connected via the pulmonary artery to the recipient’s right atrium (figures 1A–D).2
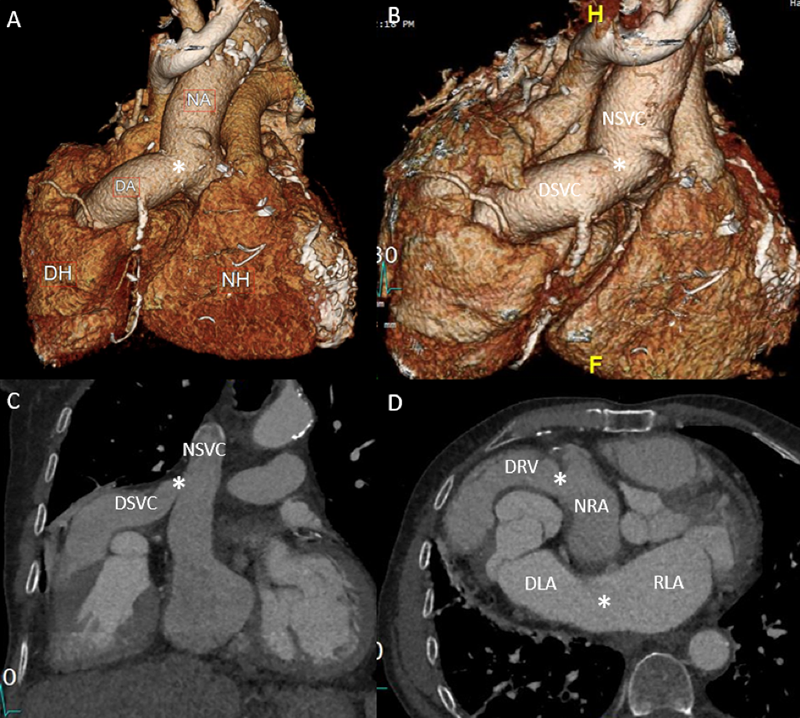
Case presentation
History
A 74-year-old man with a HHT performed 20 years ago for ischaemic cardiomyopathy presented to the transplant clinic. He recently experienced sustained native heart ventricular tachycardia (VT) which was cardioverted at a local hospital. The patient now reported a one-month history of worsening peripheral oedema and exertional breathlessness. A routine electrocardiogram (ECG) revealed recurrent native heart VT (figure 2).
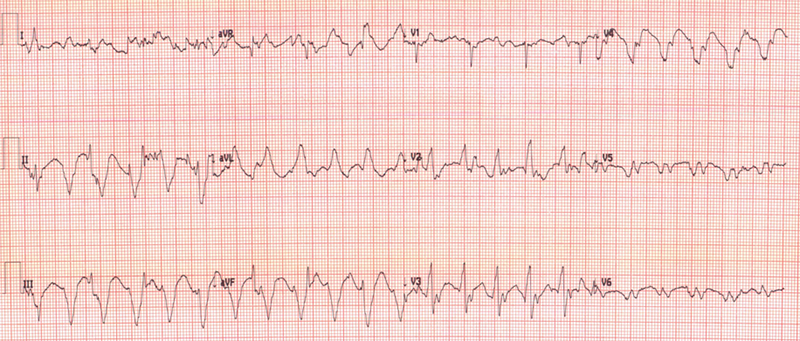
Management
Native heart sinus rhythm was restored after four direct-current cardioversion (DCCV) shocks. ECG leads were synchronised to the donor heart (on the right side), utilising the method described in Arujuna et al.,3 to isolate two superimposed rhythms. This allowed the identification of VT in the native heart and sinus rhythm in the donor heart. DCCV was then synchronised to the donor heart’s QRS complex with successful cardioversion at 360 J. Intravenous (IV) unfractionated heparin was administered to mitigate the risk of left ventricular thrombus formation. Days later, native heart VT recurred, terminating after IV amiodarone 300 mg followed by a 900 mg infusion and a further single DCCV. Twenty-four hours later, the patient developed native heart asystole, but remained stable and alert (figure 3). Amiodarone was stopped and two doses of atropine 300 mcg were given with no effect. Isoprenaline infusion was initiated and up titrated to 4 µg/hr within four hours. Repeat ECG illustrated native heart wide-complex bradycardia. Isoprenaline was gradually tapered off over 48 hours.
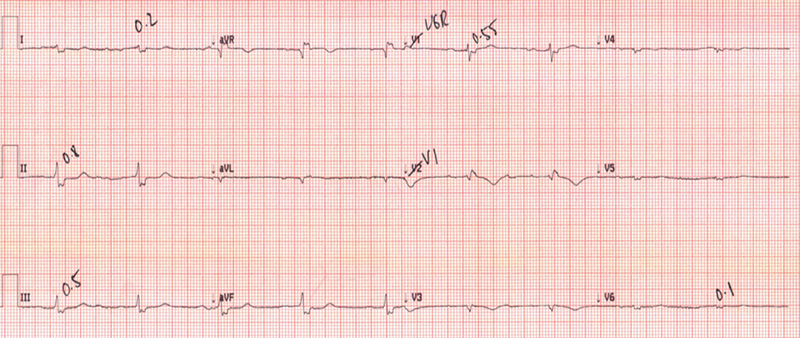
However, a third episode of native heart VT occurred, followed by an unsuccessful ablation attempt. Lignocaine infusion was uptitrated from 1–4 mg/min, which successfully reverted the VT but the patient developed a second episode of asystole lasting greater than 24 hours. A pacemaker was inserted, and oral mexiletine 100 mg three times daily replaced intravenous lignocaine for long-term management. The patient remained stable in sinus rhythm until discharge.
Discussion
Native heart VT
HHT patients tolerate native heart ventricular arrhythmias better due to the heterotopic allograft’s contribution to maintaining haemodynamics. However, over time, end-organ dysfunction can develop due to the loss of native heart function. Management involves electrical and pharmacological measures, along with advanced ablation procedures. In this case, electrical cardioversion was attempted as the first-line treatment with transoesophageal echocardiography guidance to exclude a thrombus and ensure synchronisation of the donor heart rhythm during cardioversion.
The management of native VT was complicated by the development of native heart asystole after using amiodarone – a novel clinical situation which was encountered that has not been described in the literature.
Despite performing a VT ablation to avoid the use of pharmacological agents, the patient subsequently required pacemaker implantation for recurrent asystole. Lignocaine effectively managed subsequent episodes of VT, which was later switched to mexiletine.
Alternative options
As the severely abnormal rhythm status of this patient indicated progression in his native heart deterioration and general health (which included active malignancy), conservative management and palliation remained the default position. In addition, the patient remained haemodynamically stable and relatively asymptomatic throughout.
Thrombosis of the native heart
Severe aortic regurgitation created blood flow into the native left ventricle and blood flow continued from the donor pulmonary artery into the native right-sided system. This prevented stagnation of blood within the native heart, thus reducing the risk of thrombus formation.
Pacing asystole
Pacing for native heart asystole has limited evidence for its benefit.4 However, in this unique case, electrical capture was achieved despite over 24 hours of asystole. It is suspected that the perfusion of the native coronary arteries by the donor heart reduced the risk of myocardial muscle necrosis, allowing for successful pacing.
Conclusion
This case report described a previously undescribed case of native heart ventricular tachycardia and asystole in a patient with a HHT. The management was complex and conflicting; nevertheless, rhythm control was achieved through pacing and the use of sodium channel blockers.
Key messages
- This particular case of a heterotopic heart transplant (HHT) demonstrated the development of alternating episodes of sustained native heart ventricular tachycardia and prolonged asystole
- Generally, HHT patients tolerate native heart ventricular arrhythmias better due to the heterotopic allograft’s contribution to maintaining haemodynamics
- End-organ dysfunction can develop due to the loss of native heart function over time. Management involves electrical and pharmacological measures, paying particular attention to the unique physiology in these patients
- Although evidence for pacing in native heart asystole is limited, it was successful in this case, likely due to the sustained coronary artery perfusion from the donor heart, which may have preserved myocardial viability.
Conflicts of interest
None declared.
Funding
None.
Statement of consent
Full written patient informed consent was obtained.
References
1. Flecher E, Fouquet O, Ruggieri VG, Chabanne C, Lelong B, Leguerrier A. Heterotopic heart transplantation: where do we stand? Eur J Cardiothorac Surg 2013;44:201–6. https://doi.org/10.1093/ejcts/ezt136
2. Gaiotto FA, de Almeida Barbosa Filho AC, Tenório DF, Steffen SP, Jatene FB. Heterotopic heart transplantation as a left ventricular biological assistance: a new two-stage method proposal. Braz J Cardiovasc Surg 2020;35:986–9. https://doi.org/10.21470/1678-9741-2020-0506
3. Arujuna A, Ali K, Banner NR. Innovative electrocardiograph lead placement in heterotopic heart transplant patients undergoing cardioversion of ventricular arrhythmias. J Heart Lung Transplant 2016;35:1146–8. https://doi.org/10.1016/j.healun.2016.06.009 [Epub online ahead of print]
4. Knowlton AA, Falk RH. External cardiac pacing during in-hospital cardiac arrest. Am J Cardiol 1986;57:1295–8. https://doi.org/10.1016/0002-9149(86)90207-9
