Protection against the increased risk of sudden cardiac death (SCD) due to ventricular arrhythmias is offered by the implantation of cardiac defibrillators. A life-expectancy of less than one year is usually a contraindication to the implantation of these devices. We evaluated the outcomes of all those who received defibrillator implantation for any clinical indication at our centre in the same year that early (<12 months) death notifications occurred.
This is a single-centre retrospective study on the outcomes of all patients who had a transvenous defibrillator implant in 2015. All transvenous defibrillator devices implanted for both primary and secondary prevention of SCD were included. Patient demographic data and device data were studied.
Data from 235 patients were analysed. In a follow-up period of 66.2 ± 3.8 months, 77 (32.8%) of the study cohort died; 20 (8.5%) of these patients died less than 12 months post-implant. None of the deaths were directly arrhythmia related. Factors that were significant in predicting mortality included age and ejection fraction <35% (p<0.01). From a pre-procedure biomarker perspective, an increased red cell distribution width (RDW) was strongly associated with early mortality risk on univariate and multi-variate analysis (p<0.001). Receiver operator characteristics (ROC) curve analysis found that the optimal cut-off for RDW was 14.75% (area under curve 0.75; sensitivity 0.69; specificity 0.77; p<0.001).
In conclusion, there are limitations in fully assessing patient prognosis despite current guidance. Universal clinical frailty scores that incorporate biomarkers may be helpful in enhancing this pre-assessment process to improve the evaluation of the risk of early non-arrhythmic-related death for implantable cardioverter defibrillator candidates.
Introduction

There is a wide range of cardiac conditions that may significantly increase the risk of sudden cardiac death (SCD). They range from ischaemic heart disease to inherited cardiac conditions, such as hypertrophic obstructive cardiomyopathy, and these may be in the setting of primary or secondary prevention. Potential device candidates are expected to have a reasonable life-expectancy of at least one year.1 While clear guidelines help cardiologists and their multi-disciplinary teams to steer the right patients forward for a defibrillator device and to the right type, it is recognised that it may be difficult to assess the frailty of a patient fully and to predict those with a life-expectancy of less than 12 months. The pacing department at our centre received notifications of deaths earlier than expected after defibrillator implantation, which as part of the clinical audit, led to the review of these cases. Factors found to be associated with an increased risk of early mortality (<12 months) were scrutinised. These outcomes were compared with established guidelines and knowledge.
Purpose
To evaluate the short- and long-term outcomes of all those who received defibrillator implantation for any clinical indication at our centre in the same year that earlier-than-expected death notifications occurred. Factors found to be associated with early mortality were then analysed for the feasibility of working into a clinical score on prognosis.
Method
The clinical audit and review of unexpectedly early deaths after transvenous defibrillator implantation occurred in 2015. We reviewed patient records of all patients who underwent defibrillator implantation in 2015 at St. George’s Hospital, London, and subsequently gathered and studied 12-month and five-year outcome data, including demographic data, indication for implantation, peri-procedure ejection fraction, pre- and post-procedure laboratory results, comorbidities and follow-up outcomes, including device therapy.
We included single- and dual-chamber implantable cardiac defibrillators (ICD) as well as cardiac resynchronisation therapy-defibrillators (CRT-D) and upgrades of pre-existing pacemakers to defibrillators. No underlying cardiac conditions were excluded, and we included devices inserted for both primary and secondary prevention. Simple defibrillator generator replacement and specific lead-related procedures were excluded.
Statistics
Analysis was performed with IBM SPSS statistical software (version 22.0, IBM SPSS Statistics, NY, USA). Univariate analyses of dichotomous, categorical, and continuous variables were performed to determine similarities or differences between patients who died or not within our follow-up period following defibrillator implantation. Tabulations of dichotomous and categorical data for comparisons using the Chi-square test or Fisher’s exact test. The distribution of continuous variables was assessed for normality with Shapiro-Wilk test, and Mann-Whitney U tests were used to compare both groups. Cox regression proportional hazards model was used to assess variables that predict mortality over time.
Several models of multi-variate regression analyses were made with all independent categorical and continuous variables that appeared significant (p<0.05) for mortality. Using a forward stepwise likelihood ratio regression analysis, the most significant variables to predict mortality were determined.
A score model for mortality was developed as a logistic probability unit [z = logit (p) = log e (p/1–p); p = 1/1+e–z]; and the area under the receiver operating characteristic (ROC) curve was generated. The best cut-off point of the scoring model was identified for further comparisons, and the likelihood ratio of a positive test result was calculated. The Holm-Sidak method was used to compare group differences based on additive scores.
Results
Table 1. Patient demographics and relative risks for <12 month mortality
| p value for <12 month mortality | Odds ratio | |
| Diabetes | 0.0002 | 3.87 |
| Age >70 years | 0.0002 | 4.08 |
| Dialysis dependence | 0.0004 | 10.12 |
| Heart failure indication | 0.004 | 11.03 |
| Neurological disease | 0.012 | 3.92 |
| Dual pathology of arrhythmia and heart failure | 0.016 | NA |
| Ejection fraction <35% | 0.018 | 2.8 |
| Absence of underlying sinus rhythm | 0.03 | 2.21 |
| STEMI/NSTEMI | 0.04 | 2.17 |
| Respiratory disease | 0.071 | 0.071 |
| CABG/PCI <3 months prior to device | 0.27 | 0.27 |
| Post-procedure creatinine increment | 0.71 | 0.71 |
| Active malignancy | 1 | NA |
| Key: CABG = coronary artery bypass graft; NA = not applicable; NSTEMI = non-ST-elevation myocardial infarction; PCI = percutaneous coronary intervention; STEMI = ST-elevation myocardial infarction | ||
Two hundred and thirty-five patients had a transvenous defibrillator implanted in 2015, excluding generator substitutions and lead removal and replacement procedures. One-year and up to five-year outcomes data pertaining to 235 patients were analysed.
Patient demographics
The median age of patients was 67 years, with a predominantly male population (76%). In a follow-up period of five years from the date of device implant (or 66.2 ± 3.8 months), 77 (32.7%) of the study cohort died, including 20 (8.5%) who died less than 12 months post-implant. None were arrhythmia related. No breach in recognised screening practice or clinical indications for a device occurred (table 1).
Patient variables associated with an increased risk of early mortality included raised creatinine (ROC cut-off >100 µmol/L), pre-procedure haemoglobin (Hb) <130 g/L, greater age (ROC cut-off ≥70 years), diabetes mellitus, and prior neurological disease such as ischaemic or haemorrhagic stroke (p values are <0.001) (supplementary table 3).
Device therapy and cardiac risk factors
ICD anti-tachycardia pacing (ATP) therapy and shock therapy events were not associated with subsequent mortality (p=0.34 and p=0.56, respectively). There was no statistical significance for secondary versus primary prevention (p=0.56). Cardiac-specific risk factors included a history of sustained ventricular tachycardia (VT) on device checks, cardiac resynchronisation therapy (CRT) implantation, ejection fraction (EF) <35%, absence of underlying sinus rhythm and previous ST/non-ST segment elevation myocardial infarction (STEMI/NSTEMI) (respective p values 0.0001, 0.0017, 0.018, 0.03, 0.04).
Pre-implant laboratory pathology
Increased red cell distribution width (RDW) was significantly associated with increased mortality in univariate (p<0.001) and multi-variate analysis (p=0.04). ROC curve analysis found that the best cut-off was 14.75% (AUC 0.75; sensitivity 0.69; specificity 0.77; p<0.001) (figure 1, table 2, and supplementary table 3).
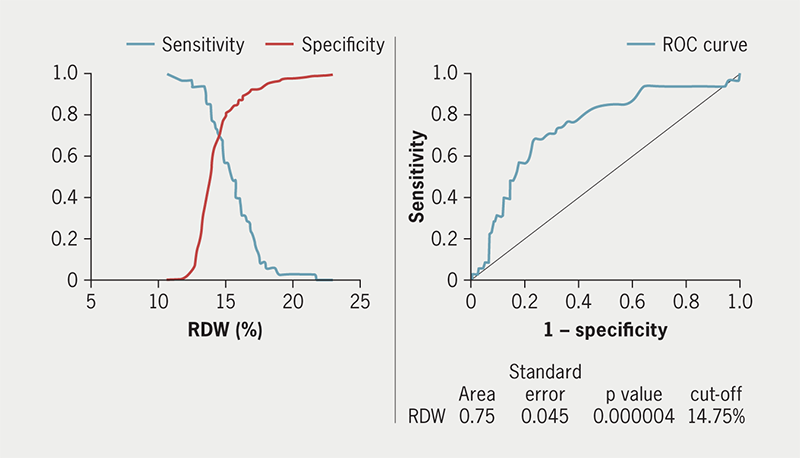
Table 2. Risk factor univariate and multi-variate analysis
| Risk factor | Univariate analysis | Multi-variate analysis | ||
| p value for mortality <12 months | Odds ratio | p value for mortality <12 months | Hazard ratio | |
| RDW >14.75% | <0.000001 | 7.26 | 0.048 | 2.32 |
| BNP >9,105.5 ng/L | <0.000001 | 13.5 | NA | NA |
| Creatinine >100 µmol/L | 0.000001 | 7.48 | 0.039 | 2.72 |
| Hb pre-procedure <130 g/L | 0.00009 | 4.41 | 0.49 | 1.38 |
| History of sustained VT recorded on device | 0.0001 | 5.34 | 0.001 | 4.68 |
| Diabetes | 0.0002 | 3.87 | 0.14 | 1.72 |
| Age >70 years | 0.0002 | 4.08 | 0.1 | 2.05 |
| Dialysis dependent | 0.0004 | 10.12 | – | – |
| Heart failure indication | 0.004 | 11.03 | – | – |
| Neurological disease (CVA, hypoxic brain injury) | 0.012 | 3.92 | 0.025 | 2.94 |
| CRT-D (vs. ICD) | 0.017 | 2.40 | – | – |
| Ejection fraction <35% | 0.018 | 2.8 | 0.56 | 0.75 |
| Dual-chamber device | 0.019 | 2.58 | – | – |
| Absence of underlying sinus rhythm | 0.03 | 2.21 | 0.83 | 0.92 |
| STEMI/NSTEMI | 0.04 | 2.17 | 0.48 | 0.82 |
| Key: BNP = B-type natriuretic peptide; CRT-D = cardiac resynchronisation therapy-defibrillator; CVA = cerebrovascular accident; Hb = haemoglobin; ICD = implantable cardioverter defibrillator; NA = not applicable; NSTEMI = non-ST-elevation myocardial infarction; RDW = red cell distribution width; STEMI = ST-elevation myocardial infarction; VT = ventricular tachycardia | ||||
Due to the range of indications for ICD implantation, not all patients had a recorded B-type natriuretic peptide (BNP) measurement at pre-assessment; it is also not a routine measurement at the time of device implantation. Only 109 patients (43.6%) had a BNP measurement. Of those patients with BNP measurement before implant, a raised BNP was statistically significant, with ROC analysis determining 9,103.5 ng/L as a cut-off (p<0.001). The time of its measurement was not standardised; whereas all other blood tests were performed within one-week pre-implantation, the interval from BNP measurement to implantation ranged from one to 48 weeks. BNP measurements were, therefore, excluded from multi-variate analysis. RDW was the strongest predictor of patient outcomes among the variables studied.
RDW and association with age, left ventricular function, haemoglobin and renal function
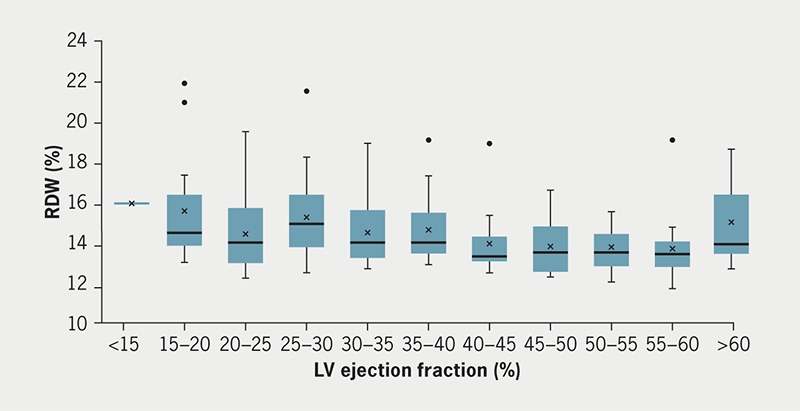
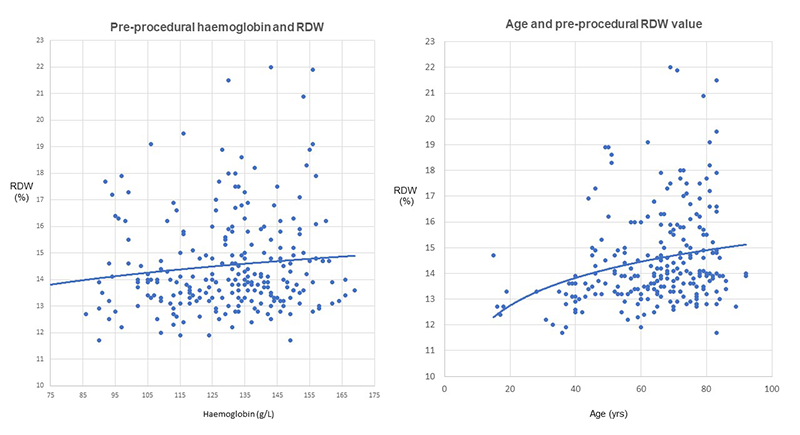
RDW was not significantly different when simply comparing all those with impaired cardiac function (EF <55%) to those with normal cardiac function (EF >55%) (supplementary table 4 and figure 2). But on closer inspection of the RDW values across the range of EF values (supplementary table 4), a cut-off of EF <40% was found to be significantly different, 14.2% (interquartile range [IQR] 13.5–15.9%) versus 13.6% (IQR 13.1–14.4%), p<0.001. An age of >70 years was associated with a slightly higher RDW value, but haemoglobin below the lower limit of normal for women (<12 g/L) was not significant (p>0.05) (supplementary figure 1). Impaired renal function pre-implant with a creatinine of >100 µmol/L was associated with significantly higher RDW values than in the group with <100 µmol/L 14.8% (IQR 13.9–16.35) versus 13.6% (IQR 13.15–14.55) (supplementary table 4).
Five-year outcomes
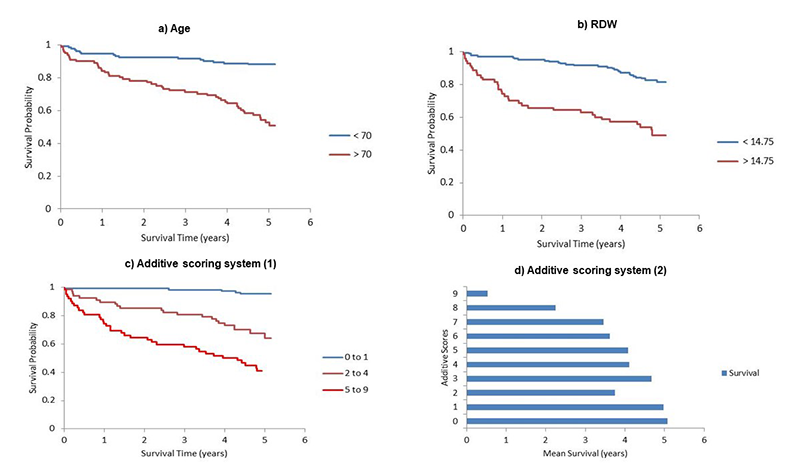
A review of the clinical outcomes of the study patients at five years was conducted using inter-hospital electronic records. Of those that survived >12 months, but died within the subsequent four-year follow-up period, the mean time to death was 38.2 months (range 12.1–48 months). In total, of the 235 patients included in this study, 77 died (32.8%) within five years. The cause of death was not directly arrhythmia related in these cases. Causes included end-stage heart failure, influenza and multi-organ failure and COVID-19 pneumonia. A Kaplan-Meier survival analysis shows a clear difference in outcomes with an RDW cut-off value of 14.75% (supplementary figure 2, panel b).
Feasibility of an RDW-based prognostic scoring system
From multi-variate analysis, we were able to design a mortality risk additive scoring system. With five variables of RDW, creatinine, age, diabetes mellitus and neurological disease (the strongest significant variables in this study), and allocation of a weighted score of one or three, the total potential maximal potential score was nine. A total additive score of nine is associated with a one-year mortality risk of 68.8% (supplementary figure 2: panels c and d, supplementary table 1, supplementary tables 5–7).
Discussion
At our centre, we tried to observe the recommendation that ICD therapy should not be used in patients who will not survive at least one year.1 Our observed 8.5% one-year mortality post-ICD implantation suggests that our ability to prognosticate can be limited. None of the earlier-than-anticipated deaths post-implant had breached recognised guidelines in patient screening and indication. If we had foreseen these 20 early deaths, we would have avoided implanting these devices, saving the patients the risk and discomfort of the procedure, and saving our healthcare system more than £150,000 in average hardware costs at the list price.
Patient demographics
This study confirms known mortality predictors, including advanced age, diabetes, prior myocardial infarction (MI), renal failure and poor left ventricular (LV) systolic function as markers of high risk. RDW now joins this list as an established independent predictor of one-year and five-year mortality. An attempt at a clinical model to predict the risk of early mortality after primary prevention ICD implant was conducted by Bilchick et al.,2 which included similar indices and had consistent positive findings in their validation cohort. Their ‘SHOCKED’ predictors included: age >75 years, New York Heart Association (NYHA) III, renal failure, atrial fibrillation (AF), chronic obstructive pulmonary disease (COPD), EF <20% and diabetes mellitus. No biomarkers were combined into their model. In our study, on univariate and multi-variate analysis of all studied indices, RDW was the strongest marker for all-cause mortality in the first year after ICD implantation.
BNP
Of the subgroup of patients who had a BNP measurement, a raised BNP was found to be significantly related to one-year mortality (p<0.001). This confirms the increased mortality association in those with a heart failure diagnosis. ROC analysis area under the curve was used to delineate a BNP cut-off, and this was found to be at 9,103.5 ng/L. This figure is far above the upper limit of normal for the BNP range in our lab (400 ng/L). The result is significantly skewed as BNP measurements were only performed in cases where it was clinically indicated, e.g. in acute heart failure or prior to defibrillator implant. Only 109 of the 235 patients had a BNP measurement within 12 months pre-implant, so BNP assessment could not be included in the multi-variate analysis as it was not measured in an elective setting similar to the other parameters.
RDW
In our study, the best cut-off value for RDW was 14.75% (ROC analysis, AUC 0.75; sensitivity 0.69; specificity 0.77; p<0.001). Our analysis has been able to identify a discrete cut-off value that can be put into adjusted use in future scoring systems. RDW was included in our study analysis due to the knowledge of its prognostic significance from previous studies, and that it is a surrogate marker of poor physiological status.3–13 Results indicated that RDW was independent of haemoglobin levels (supplementary table 4). No study to date has looked at the significance of RDW on mortality across all patients receiving any type of transvenous defibrillator implant. Dabbah et al.11 studied the combined outcomes of mortality and first appropriate ICD therapy, and found that those with the third highest quartile of RDW values were associated with increased risk of the combined outcomes, and that the patients in this group were older with more comorbidities.
RDW and its association with other variables
In this study, RDW was not linked to low levels of haemoglobin, but higher RDW values were associated with advanced age, LV systolic function of less than 40% and with renal impairment with a creatinine >100 µmol/L. These observations were in keeping with previous assessments that RDW is linked to clinical parameters of patient frailty with advanced age and comorbidities.
Studies on early mortality post-ICD implant
A recent observational study by Garcia et al. (the DAI-PP program),14 looked at predictors of early mortality post-ICD implant from the implant era of 2002–2012. Overall, 4.2% died <12 months from the time of device implant, and it found that older age (>70 years), functional NYHA class III or more and AF were significant factors associated with <12 months mortality. If all three risk factors were present, the risk of early mortality was over 20%. Again, as with our study, those who died early had low device therapy events compared with those who survived >12 months from the implant, suggesting early deaths were not directly arrhythmia related, and again, confirming our limitations in assessing overall increased risk of early mortality from non-arrhythmic causes.
In a registry study15 of those who received CRT-D implants only, early mortality was predicted by a history of percutaneous coronary intervention (PCI) and peripheral vascular disease, but in this study, early mortality was defined as within three years from the date of the implant. Only the study by Zhang et al.16 investigated combined clinical and serum-based biomarkers of early mortality. Only those who received de novo primary prevention ICD implants were included. Combining serum markers of troponin, tumour necrosis factor alpha and BNP enhanced the clinical predictive model from an AUC of 0.77 to 0.82.
Five-year outcomes after ICD implant
A significant proportion (24.3%) of the study population died between 12 months and five years, with overall 32.8% at the end of follow-up. We must bear in mind that the tail end of the follow-up coincided with the first wave of the COVID-19 pandemic. The study by Madhavan et al.17 reported a mortality of 25% in five years after a generator replacement, and this was approximately 32% in the ALTITUDE Survival study.18 In these studies, all ICD (and CRT-D in ALTITUDE Survival) recipients were included, similar to ours. The cause of death was not found to be directly device or arrhythmia related in any of the cases in these studies. End-stage heart failure, ischaemic heart disease and chest sepsis were the common causes found. Kaplan-Meier analysis demonstrated that RDW values greater than 14.75% were associated with poorer long-term survival outcomes, with the disparity greater than the age comparison analysis (supplementary figure 2: panels a and b).
Use of RDW in a prognostic scoring system
It was a surprise on the initial review of the pre-assessment biomarkers that RDW was the strongest independent prognostic biomarker. But in fact, literature has consistently demonstrated the poor prognostic outcomes of raised RDW across a range of cardiovascular diseases (supplementary table 1).3–13 There is clinical value in the measurement of RDW, and this information is widely available due to its inclusion in the full blood count. In our cohort of patients, we have captured the RDW value at a single point in time, pre-defibrillator implantation, a form of cardiac intervention. It is thought that the RDW value is a surrogate of the physiological status of the patient: RDW represents a systemic marker of frailty.
In clinical practice, where the appropriateness of defibrillator implantation for a patient is unclear due to age and concurrent comorbidities or frailty, we currently use multi-disciplinary team discussions, in addition to informed conversations with the patient and their relatives, in order to make a pragmatic decision about implantation. It is in these situations where an additional adjunctive objective scoring assessment may be of clinical benefit.
From the statistically significant variables collated in our study, we have devised a preliminary additive scoring system (with a 0–9 point scale) (supplementary figure 2: panels c and d, and supplementary tables 4–7). The established prognostic variables include age, LV systolic function, renal function and neurological disease in addition to RDW. The first three variables are already incorporated into current commonly used risk score calculators, such as EUROSCORE II and GRACE. The Holm-Sidak method was used to compare groups based on scores, and this showed statistically significant differences in survival rates across different score comparisons (supplementary table 6).
Not only does our data suggest that the potential to design and test a new frailty scoring system using RDW is feasible, but there is also the possibility of modifying existing scoring systems to include RDW and to test if there is enhanced sensitivity and specificity in quantifying patient ‘early mortality’ risk.
There have been studies looking at the application of well-known cardiac scoring systems: Meune et al.19 conducted a study looking at the performance of the GRACE score in the era of high-sensitivity troponin I (hs-TnI) and BNP. The GRACE score on its own had an AUC of 0.87 and 0.88 for in-hospital and one-year mortality, respectively, but the addition of hs-TnI to the score did not increase these values. The addition of BNP only increased one-year mortality values. A similar type of study could be conducted in the ICD cohort where RDW is incorporated into existing scoring systems.
Summary
The initiation of this project began in response to the pacing department notifications of earlier-than-expected deaths after a defibrillator implant at our centre. The purpose was to ensure that our ability to screen was in line with recognised guidelines. This has been fulfilled with no anomalies identified in the screening process. The increased early mortality rate in this particular year, compared with literature, was observed in older patients with comorbidities with the additional finding of RDW as a significant biomarker. Our other findings on factors associated with early mortality are mostly in line with established device studies. Further work may help to close the gap in our ability to predict early non-arrhythmic-related death.
Limitations
We performed a retrospective study, therefore, missing data limited the full analysis of certain variables, particularly the inability to include BNP in a multi-variate analysis. To provide further support for the study findings, a multi-centre approach and a longer duration of study would be required with additional prospective data collection of all clinical and biomarker variables for all patients undergoing ICD implantation. The scoring system proposed in this study was to determine the feasibility of incorporating RDW as a biomarker among prognostic clinical variables based on multi-variate analysis of this study’s dataset; however, it is not at a stage for clinical use. Further work is required to refine and prospectively test this, or indeed any novel scoring system, against the standard approach with clinical assessment alone and/or with established risk scores.
Conclusion
There are limitations in fully assessing patient prognosis despite current guidance. Universal clinical frailty scores that incorporate biomarkers may be helpful in enhancing this pre-assessment process. In this study, increased RDW is a strong independent predictor of early mortality in the cohort of patients receiving defibrillator implantation. RDW can potentially be used to help with risk stratification in cases where the benefit of ICD implantation is uncertain due to comorbidity increasing the risk of early non-arrhythmic-related death.
Key messages
- There are limitations in predicting early non-arrhythmic related death in potential implantable cardioverter defibrillator (ICD) recipients
- Early deaths in this study, as with others in literature, indicate that clinical causes behind non-arrhythmic deaths are related to age and comorbidities, i.e. overall frailty
- Incorporation of independent prognostic biomarkers into clinical risk scores may be a future direction for research aiming to improve the assessment of frailty and risk of early non-arrhythmic related death, and so avoid unnecessary procedures that impact the patient nearing the end of life and healthcare costs
Conflicts of interest
LWML previously received research support from Attune Medical (Chicago IL). MMG has received research funding from Attune Medical, Medtronic and has acted as a consultant for Medtronic and for Cook Medical. ZA, OV, GS, RW, JV, SJ, PK, NS: none declared.
Funding
None.
Study approval
St. George’s Hospital London institutional approval was obtained.
Data sharing statement
The corresponding author will share data related to this study upon reasonable request. Corresponding author can be contacted by email. All supplementary data are available from the authors on request. Supplementary tables are:
Supplementary Table 1. Red cell distribution width and cardiovascular studies
Supplementary Table 2. A nine-point additive scoring system based on logistic regression analysis
Supplementary Table 3. Receiver operating characteristics analysis of age, RDW, haemoglobin, renal function
Supplementary Table 4. Relationship between RDW and patient variables
Supplementary Table 5. Nine-point additive scoring and survival
Supplementary Table 6. Holm-Sidak post hoc comparisons between groups based on different additive scores
Supplementary Table 7. Mortality data grouped by additive scoring
Acknowledgement
We are grateful for the support of the pacing department at St. George’s Hospital, Tooting.
References
1. Brignole M, Auricchio A, Baron-Esquivias G et al.; the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy. Developed in collaboration with the European Heart Rhythm Association (EHRA). Rev Esp Cardiol (Engl Ed) 2014;67:58. https://doi.org/10.1016/j.rec.2013.11.003
2. Bilchick KC, Stukenborg GJ, Kamath S, Cheng A. Prediction of mortality in clinical practice for medicare patients undergoing defibrillator implantation for primary prevention cardiac death. J Am Coll Cardiol 2012;60:1647–55. https://doi.org/10.1016/j.jacc.2012.07.028
3. Das Gupta A, Hegde C, Mistri R. Red cell distribution width as a measure of severity of iron deficiency in iron deficiency anaemia. Indian J Med Res 1994;100:177–83.
4. Barrett AN, Saminathan R, Choolani M. Thalassaemia screening and confirmation of carriers in parents. Best Pract Res Clin Obstet Gynaecol 2017;39:27–40. https://doi.org/10.1016/j.bpobgyn.2016.10.015
5. Lian Y, Shi J, Nie N, Huang Z, Shao Y, Zhang J et al. Reticulocyte hemoglobin equivalent (Ret-He) combined with red blood cell distribution width has a differentially diagnostic value for thalassemias. Hemoglobin 2019;43:229–35. https://doi.org/10.1080/03630269.2019.1655440
6. Cai Y, Liu D, Cui J et al. Diagnostic accuracy of red blood cell distribution width to platelet ratio for predicting staging liver fibrosis in chronic liver disease patients: a systematic review and meta-analysis. Medicine (Baltimore) 2019;98:e15096. https://doi.org/10.1097/MD.0000000000015096
7. Celikyurt U, Agacdiken A, Sahin T, Kozdag G, Vural A, Ural D. Association between red blood cell distribution width and response to cardiac resynchronization therapy. J Interv Card Electrophysiol 2012;35:215–18. https://doi.org/10.1007/s10840-012-9694-1
8. Duchnowski P, Hryniewiecki T, Stoklosa P, Kuśmierczyk M, Szymański P. Red cell distribution width as a prognostic marker in patients undergoing valve surgery. J Heart Valve Dis 2017;26:714–20. https://doi.org/10.1093/icvts/ivx216
9. Abrahan LL 4th, Ramos JDA, Cunanan EL, Tiongson MDA, Punzalan FER. Red cell distribution width and mortality with acute coronary syndrome: a meta-analysis on prognosis. Cardiol Res 2018;9:144–52. https://doi.org/10.14740/cr732w
10. Güngör B, Özcan KS, Erdinler I et al. Elevated levels of RDW is associated with non-valvular atrial fibrillation. J Thromb Thrombolysis 2014;37:404–10. https://doi.org/10.1007/s11239-013-0957-1
11. Dabbah S, Chertin L, Khateeb A, Rosenfeld I, Suleiman M, Halabi M. Red cell distribution width predicts death and appropriate therapy in patients with implantable cardioverter defibrillator: a simple measurement with prognostic value in a variety of diseases, may help in better selection of patients who will benefit the most from this device. Pacing Clin Electrophysiol 2017;40:1384–8. https://doi.org/10.1111/pace.13226
12. Papageorgiou N, Falconer D, Ioannou A et al. Full blood count as potential predictor of outcomes in cardiac resynchronization therapy. Sci Rep 2019;9:13016. https://doi.org/10.1038/s41598-019-49659-z
13. Lappegård J, Ellingsen TS, Skjelbakken T et al. Red cell distribution width is associated with future risk of incident stroke. The Tromso study. Thromb Haemost 2016;115:126–34. https://doi.org/10.1160/TH15-03-0234
14. Garcia R, Boveda S, Defaye P et al. Early mortality after implantable cardioverter defibrillator: incidence and associated factors. Int J Cardiol 2020;301:114–18. https://doi.org/10.1016/j.ijcard.2019.09.033
15. von Gunten S, Theuns DA, Kuhne M, Reichlin T, Sticherling C, Schaer B. Predictors for early mortality and arrhythmic events in patients with cardiac resynchronization therapy with defibrillator: a two center cohort study. Cardiol J 2019;26:711–16. https://doi.org/10.5603/CJ.a2018.0144
16. Zhang Y, Guallar E, Blasco-Colmenares E et al. Clinical and serum-based markers are associated with death within 1 year of de novo implant in primary prevention ICD recipients. Heart Rhythm 2015;12:360–6. https://doi.org/10.1016/j.hrthm.2014.10.034
17. Madhavan M, Waks JW, Friedman PA et al. Outcomes after implantable cardioverter-defibrillator generator replacement for primary prevention of sudden cardiac death. Circ Arrhythm Electrophysiol 2016;9:e003283. https://doi.org/10.1161/CIRCEP.115.003283
18. Saxon LA, Hayes DL, Gilliam FR et al. Long-term outcome after ICD and CRT implantation and influence of remote device follow-up. The ALTITUDE Survival study. Circulation 2010;122:2359–67. https://doi.org/10.1161/CIRCULATIONAHA.110.960633
19. Meune C, Drexler B, Haaf P et al. The GRACE score’s performance in predicting in-hospital and 1-year outcome in the era of the high-sensitivity cardiac troponin assays and B-type natriuretic peptide. Heart 2011;97:1479–83. https://doi.org/10.1136/hrt.2010.220988
