Fabry disease (FD), resulting from alpha-galactosidase A enzyme deficiency, remains underdiagnosed despite readily available methods for diagnosis. This multi-centre prospective survey across six tertiary centres aimed to determine the level of consideration of FD in patients presenting with left ventricular hypertrophy (LVH). LVH was defined according to the guidelines of the European Society of Cardiology (ESC) and the American College of Cardiology/American Heart Association (ACC/AHA) for the management of cardiomyopathies, where LVH is diagnosed if the left ventricular wall thickness exceeds 15 mm in any segment or exceeds 13 mm in the presence of a known diagnosis in a first-degree relative. Of 143 patients surveyed, only three with LVH underwent diagnostic testing for FD, all testing negative. Moreover, 44% of patients with LVH had an ambiguous aetiology, highlighting possible missed FD diagnostic opportunities. A discrepancy was observed between electrocardiogram (ECG) results and echocardiography or cardiac magnetic resonance (CMR) in diagnosing LVH, emphasising the need for comprehensive cardiac imaging. This project highlights the urgent requirement to amplify FD awareness, especially in patients with LVH, to ensure early intervention and better patient outcomes.
Introduction
Fabry disease is a rare X-chromosome-linked disorder that results from alpha-galactosidase A enzyme deficiency. It is broadly divided into classical (earlier onset, low enzyme activity) and non-classical (milder, later-onset and some residual activity). It is underdiagnosed despite the availability of diagnostic tests, such as blood (plasma or leucocyte alpha-galactosidase A enzyme) and genetic testing. Due to its heterogeneous nature as a multi-system disorder, Fabry disease (FD) is rarely considered. Patients often present with non-specific symptoms, such as fatigue and gastrointestinal symptoms akin to irritable bowel, taking an average of 10–15 years to reach a conclusive diagnosis.1,2 Although, individually, rare diseases are uncommon, cumulatively they effect one in 17 people at some point in their lifetimes. There are over 7,000 rare diseases, affecting an estimated 3.5 million people in the UK.3 While most rare diseases have no treatment, FD benefits from available effective treatment regimens including enzyme replacement and oral chaperone therapy.
Even though FD is a rare condition, its early detection and diagnosis are of paramount importance because timely intervention can significantly improve the quality of life, prevent severe complications, and enhance the effectiveness of available treatments. Delayed or missed diagnosis can lead to irreversible damage and reduced life-expectancy. Cardiac manifestations of FD are the most common cause of mortality, and, in adults, the earliest cardiovascular manifestation of FD that can be picked up at the bedside is left ventricular hypertrophy (LVH).4–6 This typically occurs in the third to fourth decades of life, and the prevalence of FD in patients with unexplained LVH is 1–3%.7–10 Early detection of FD is crucial, as initiating FD-specific treatment promptly can slow down its multi-organ effects, with limited evidence highlighting that this may also be the case for LVH. Conversely, treatments have proven less effective when introduced during the advanced stages of the disease, marked by fibrosis and pronounced LVH.11
The prevalence of FD is likely underestimated,12,13 and prior studies have shown an increase in the detection of patients with FD through screening of populations with hypertrophic cardiomyopathy.7-9,14 LVH was determined based on the criteria set by the European Society of Cardiology (ESC)15 and the American College of Cardiology/American Heart Association (ACC/AHA),16 where a diagnosis of LVH is made if the left ventricular wall thickness is greater than 15 mm in any segment, or more than 13 mm when there is a known diagnosis in a first-degree relative. Therefore, the aim of this project was to explore whether FD was considered as a possible diagnosis in the presence of LVH, and to evaluate it against recent guidelines on the investigation of LVH of unknown aetiology by Linhart et al.11
Materials and method
We conducted a three-week multi-centre prospective survey across six centres in England, Northern Ireland, and Scotland, to provide a snapshot of LVH presentations in outpatient clinics, including whether differential diagnoses, such as FD, were considered. Each centre registered with their relevant local departments. Anonymised data were collected from patients seen in cardiology clinics, excluding those with a confirmed diagnosis of FD.
Testing for FD was determined by each local centre involved in the study according to standard of care. This testing typically consisted of both alpha-galactosidase A enzyme assays and targeted genetic sequencing. While enzyme testing was used to diagnose FD, particularly in male patients, genetic sequencing was employed to confirm the diagnosis, especially in females, where enzyme levels may not always be indicative of the disease due to X-chromosome inactivation.
Broad genetic panels, which might incidentally detect Fabry mutations without specific clinical suspicion, were not used. Instead, where genetic testing was conducted, most centres employed targeted genetic panels specifically designed for hypertrophic cardiomyopathy (HCM) and FD. This approach ensured a focused evaluation for FD, while considering other genetic causes of LVH.
Only aggregated data were combined between the trusts. Data included the presence of LVH on electrocardiogram (ECG), cardiovascular magnetic resonance (CMR), or echo; signs/symptoms and past medical history associated with FD, such as renal dysfunction (including proteinuria) and gastrointestinal symptoms such as irritable bowel; and whether a diagnosis of FD was considered and investigated. Categorical variables are expressed as numbers and percentages, while continuous variables are presented as means ± standard deviation (SD) or medians with interquartile ranges (IQR), as appropriate. Statistical significance was set at p<0.05. The statistical analyses were conducted using the Python programming language.
Results
Table 1. Summary of main findings
| Variable | Percentage (n) N=143 |
| Sex | |
| Male patients | 48% (70) |
| Female patients | 52% (73) |
| Ethnicity | |
| White | 66% (95) |
| Asian/Asian British | 26% (11) |
| Black/Black British | 4% (6) |
| Clinic type | |
| Telephone clinics | 63% (90) |
| Face-to-face clinics | 69% (99) |
| Clinical findings | |
| Patients with LVH on ECG | 18% (26) |
| Patients with LVH on echo | 30% (36) |
| Patients with LVH on CMR | 46% (12) |
| Investigations | |
| Patients tested for FD | 9% of those with LVH (3) |
| Patients with unclear LVH diagnosis | 44% of those with LVH (15) |
| Associated conditions | |
| Patients with renal failure | 12% (17) |
| Patients with gastrointestinal symptoms | 4% (5) |
| Patients with T-wave inversion on ECG | 15.4% (22) |
| Key: CMR = cardiac magnetic resonance; ECG = electrocardiogram; FD = Fabry disease; LVH = left ventricular hypertrophy | |
A total of 143 responses were collected by six tertiary centres. Table 1 summarises the findings. Median completion time (available for 123 entries) of data capture was four minutes per clinic (range 1–12 minutes, IQR 2–4 minutes). The patients had a near equal male (48% n=70) to female (52% n=73) distribution with a median age of 60.5 (IQR 41–71) years for males and 60 (IQR 45–71) years for females. There was predominantly white ethnicity 66% (n=95), and 26% (n=11) were Asian/Asian British and 4% (n=6) Black/Black British. The responses were from both telephone (63% n=90) and face-to-face (69% n=99) clinics. The most common indication (39% n=56) for referral to clinic was investigation of a cardiac symptom, such as shortness of breath and chest pain. Clinic types included: heart failure, cardiomyopathy, inherited cardiac conditions and general cardiology, with a combination of physician- and specialist nurse-led clinics (figure 1).
Of the total 143 cohort, 18% (n=26) had LVH on ECG meeting Sokolov or Cornell criteria, confirmed by echocardiogram or CMR (mean maximum wall thickness [MWT] of 18 mm, n=11 received both echo and CMR). Of the LVH picked up on ECG, 50% (n=13) also had hypertension and 19% (n=5) had co-existing renal failure. All (100%) went on to have an echocardiogram, which confirmed the presence of LVH in 62% (n=16). In addition, 46% (n=12) went on to have the LVH confirmed via CMR. Of the LVH noted on CMR, 67% (n=8) also had associated late gadolinium enhancement of the basal inferolateral wall. Of the 30% (n=36) of patients that had LVH picked up on echocardiogram, the most common indication for this was for HCM screening (53% n=19), however, the second most common reason for the echocardiogram request was to investigate shortness of breath (28% n=10), followed by incidental finding of LVH on ECG or echo (17% n=6). There were 36% with LVH on echo (n=21) that did not have a CMR. As the LVH was detected using various diagnostic methods (ECG, echocardiography, and CMR), we excluded patients who had LVH diagnosed on multiple imaging modalities to prevent duplication. Therefore, the total number of unique patients with LVH diagnosed on any modality was 34.
Only three patients with LVH picked up on any modality were investigated for FD, with negative results. In 44% of patients with LVH who were not screened for FD (n=15), the diagnosis was unclear with probable HCM (negative genetic panel) or likely hypertensive heart disease given as the diagnosis, indicating missed diagnostic opportunities for FD.
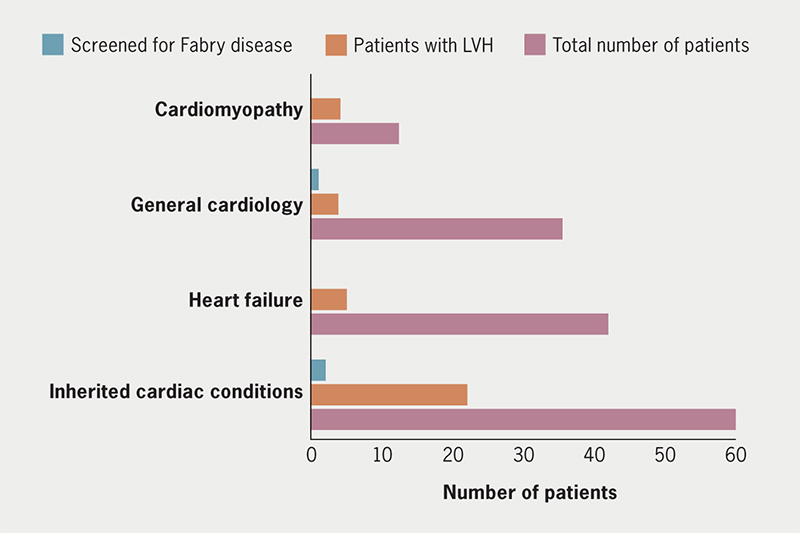
In our survey spanning multiple centres, LVH detection via ECG, echocardiography, and CMR, showed no statistically significant gender differences. While ECG and echocardiography findings were consistent across genders, CMR results approached significance (p=0.052), suggesting a potential trend that may warrant further exploration. Overall, the diagnosis of FD was rarely considered in the context of LVH detection.
A total of 17 patients (12%) had co-existing renal failure, of these, five had LVH detected on echocardiogram and three on CMR. A further five had gastrointestinal symptoms and, of these, one had co-existing LVH and one had evidence of focal fibrosis of the basal inferolateral wall. From the total cohort of 143 patients, 22 (15.4%) displayed T-wave inversion on their ECGs. Notably, none of these patients were also reported to have LVH identified on ECG. However, delving deeper into imaging modalities, 18 out of these 22 patients (81.8%) were found to have LVH on either echocardiography or CMR. This underscores the importance of comprehensive cardiac imaging in patients with ECG abnormalities, as ECG alone may not capture the full extent of cardiac changes. Other ECG changes known to be associated with FD, such as short PR or frequent ectopics, were not reported in this cohort of patients.
Discussion
FD, a rare genetic disorder resulting from a deficiency in the alpha-galactosidase A enzyme, remains a diagnostic challenge in the realm of cardiology. As evidenced from the results of our multi-centre survey spanning six tertiary centres, the majority of patients with LVH are not being tested for FD, despite its prevalence in the population of those with unexplained LVH.11 This is particularly notable given the availability of effective treatments for FD and the potential for improved outcomes with early intervention.
The results of our analysis showed that among the patients with LVH detected through ECG, echo and CMR, only a small portion were tested for FD, with all tests returning negative. This limited testing occurs despite 44% of the patients with LVH having an undetermined diagnosis, and the survey being conducted in tertiary centres equipped with CMR and specialised tests like genetic panels. This highlights the potential for missed opportunities to diagnose FD in this patient population. Our analysis suggests that many clinicians might still be unfamiliar with the connection between LVH and FD, leading to missed or delayed diagnoses.17,18
It is known that FD age at presentation not only differs between sex but also between the classical and non-classical phenotypes.5,11,13 Studies have indicated that the initiation age for enzyme replacement therapy (ERT) is approximately 59 years for men and 60 years for women with non-classical FD.17,18 Given that the median age of participants in this survey aligns with these figures, if diagnosed, they could still potentially benefit from available treatments.
One of the striking findings in our analysis was the discrepancy between ECG results and imaging modalities like echocardiography or CMR in diagnosing LVH. While a subset of patients displayed T-wave inversion on their ECGs without a simultaneous LVH identification, a significant portion of these patients showed LVH on echocardiography or CMR. This observation underlines the importance of comprehensive cardiac imaging when assessing patients with ECG abnormalities. Sole reliance on ECG might not provide a complete picture, potentially missing cases of LVH that could be tied to FD. Furthermore, of the patients with LVH on CMR, 67% had late gadolinium enhancement (LGE) in a pattern thought to be indicative of FD.
LGE is a critical marker in diagnosing FD, as it reflects areas of fibrosis within the myocardium, which is a hallmark of advanced cardiac involvement in FD.19 The specific pattern of LGE, most commonly observed in the basal inferolateral segments of the left ventricle, is thought to result from the progressive accumulation of glycosphingolipids, leading to localised myocardial fibrosis.20,21 This pattern is distinct from that seen in other causes of LVH, such as HCM, where LGE is more diffusely distributed. The presence of LGE in FD patients is not only a diagnostic tool but also correlates with a higher risk of adverse outcomes, including heart failure and arrhythmias.21 The detection of LGE on CMR could, therefore, guide clinicians in recognising FD earlier, offering an opportunity for timely intervention with ERT, pharmacological chaperone therapy or other disease-modifying treatments.
The potential for missed diagnoses becomes notable when considering that LGE is often present before the onset of significant symptoms or overt heart failure. In our study, the significant proportion of patients with LVH who displayed LGE, but were not tested for FD, highlights the diagnostic gap. Identifying this specific LGE pattern in patients with unexplained LVH could bridge this gap, offering a non-invasive and highly sensitive method for considering FD in differential diagnoses.
It is crucial for clinicians to remain vigilant for red flags that may suggest FD in patients with unexplained LVH, as these subtle clinical clues can help direct diagnostic testing and prevent delays in diagnosis. Signs such as proteinuria, a positive family history, or characteristic rashes (angiokeratomas) are often overlooked, especially when cardiac symptoms dominate the clinical presentation. Additionally, early manifestations, such as non-specific childhood illnesses, ranging from acroparesthesias to gastrointestinal complaints, should prompt further investigation. Identifying these red flags and incorporating them into a diagnostic workup ensures that patients receive timely treatment, potentially improving outcomes and preventing the progression of multi-organ involvement (box 1).
Box 1. Key red flags for diagnosing Fabry disease (FD) in patients with left ventricular hypertrophy (LVH)
|
Our project also highlights the potential racial and ethnic disparities in the diagnosis. While the majority of patients were of white ethnicity, further research is required to ascertain if there are any barriers to care or diagnostic biases that might affect minority groups.
There are some limitations to our survey. Our sample size, although large, is unlikely to represent the entire spectrum of patients with LVH. Additionally, the prospective nature and its short duration might not capture the full range of clinical presentations and outcomes related to LVH and FD.
In conclusion, our findings highlight the pressing need to increase awareness for FD in patients presenting with LVH. Comprehensive cardiac imaging should be a standard protocol for those with ECG abnormalities, ensuring no cases of LVH, potentially indicative of FD, go unnoticed. Especially as some of the hallmarks of FD, such as LGE of the basal inferolateral wall, are only delineated through CMR. We advocate for more extensive educational initiatives targeting healthcare professionals to ensure FD remains a top differential in patients with unexplained LVH, ensuring timely intervention and improved patient outcomes. Future work should focus on broadening the sample size and understanding potential barriers to care and access to tests such as blood (alpha-galactosidase A enzyme), and genetic testing, especially among minority groups.
While this study highlights the importance of diagnosing FD in patients with unexplained LVH due to the availability of effective treatments, it is essential to acknowledge that FD is only one of many potential causes of LVH. Other conditions, such as HCM and amyloidosis, also require targeted interventions. Rather than advocating for early-stage advanced imaging for all patients, a more refined approach using risk stratification based on clinical red flags – such as proteinuria, family history, or multi-organ involvement – can help prioritise patients who may benefit most from further evaluation. This approach ensures a comprehensive differential diagnosis while optimising the use of advanced imaging to identify treatable conditions like FD early, where timely intervention may prevent end-organ damage.
Key messages
- Despite the known association between Fabry disease (FD) and left ventricular hypertrophy (LVH), the majority of patients with LVH in tertiary centres are not tested for FD
- Discrepancies between electrocardiogram (ECG) and advanced imaging methods (like echocardiography or cardiac magnetic resonance) indicate the necessity for comprehensive cardiac imaging in LVH diagnosis
- A significant portion of patients with LVH had no clear diagnosis, suggesting potential missed opportunities for FD diagnosis and treatment
- There is an imperative to enhance FD awareness and early diagnosis protocols, ensuring timely intervention and improved clinical outcomes
Conflicts of interest
BL is an employee of, and holds stock in, Amicus Therapeutics. HK, HP, MA, KL, AL, AN, KA, SN, AR, JM, DH: none declared.
Funding
This survey was conducted as part of a collaborative working arrangement with Amicus Therapeutics, which included provision of the materials needed to complete the survey and project support. The authors have not received any payments from Amicus Therapeutics.
Study approval
Each trust registered the survey with their local research/audit department and followed local guidelines with regards to data handling.
References
1. Rare Disease UK. Illuminating the rare reality. London: Genetic Alliance UK, 2019. Available from: https://geneticalliance.org.uk/campaigns-and-research/rare-disease-uk/
2. Reisin R, Perrin A, García-Pavía P. Time delays in the diagnosis and treatment of Fabry disease. Int J Clin Pract 2017;71:e12914. https://doi.org/10.1111/ijcp.12914
3. European Commission. Rare diseases. Available at: https://research-and-innovation.ec.europa.eu/research-area/health/rare-diseases_en
4. Imbriaco M, Pisani A, Spinelli L et al. Effects of enzyme-replacement therapy in patients with Anderson-Fabry disease: a prospective long-term cardiac magnetic resonance imaging study. Heart 2009;95:1103–07. https://doi.org/10.1136/hrt.2008.162800
5. Thurberg BL, Fallon JT, Mitchell R, Aretz T, Gordon RE, O’Callaghan MW. Cardiac microvascular pathology in Fabry disease. Circulation 2009;119:2561–7. https://doi.org/10.1161/CIRCULATIONAHA.108.841494
6. Weidemann F, Niemann M, Breunig F et al. Long-term effects of enzyme replacement therapy on Fabry cardiomyopathy. Circulation 2009;119:524–9. https://doi.org/10.1161/CIRCULATIONAHA.108.794529
7. Elliott P, Baker R, Pasquale F et al. Prevalence of Anderson-Fabry disease in patients with hypertrophic cardiomyopathy: the European Anderson-Fabry disease survey. Heart 2011;97:1957–60. https://doi.org/10.1136/heartjnl-2011-300364
8. Chimenti C, Pieroni M, Morgante E et al. Prevalence of Fabry disease in female patients with late-onset hypertrophic cardiomyopathy. Circulation 2004;110:1047–53. https://doi.org/10.1161/01.CIR.0000139847.74101.03
9. Monserrat L, Gimeno-Blanes JR, Marín F et al. Prevalence of Fabry disease in a cohort of 508 unrelated patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 2007;50:2399–403. https://doi.org/10.1016/j.jacc.2007.06.062
10. Palecek T, Honzikova J, Poupetova H et al. Prevalence of Fabry disease in male patients with unexplained left ventricular hypertrophy in primary cardiology practice: prospective Fabry cardiomyopathy screening study (FACSS). J Inherit Metab Dis 2014;37:455–60. https://doi.org/10.1007/s10545-013-9659-2
11. Linhart A, Germain DP, Olivotto I et al. An expert consensus document on the management of cardiovascular manifestations of Fabry disease. Eur J Heart Fail 2020;22:1076–96. https://doi.org/10.1002/ejhf.1960
12. Mehta A, Beck M, Elliott P et al. Enzyme replacement therapy with agalsidase alfa in patients with Fabry’s disease: an analysis of registry data. Lancet 2009;374:1986–96. https://doi.org/10.1016/S0140-6736(09)61493-8
13. Linhart A, Kampmann C, Zamorano JL et al. Cardiac manifestations of Anderson-Fabry disease: results from the international Fabry outcome survey. Eur Heart J 2007;28:1228–35. https://doi.org/10.1093/eurheartj/ehm153
14. Seo J, Kim M, Hong GR et al. Fabry disease in patients with hypertrophic cardiomyopathy: a practical approach to diagnosis. J Hum Genet 2016;61:775–80. https://doi.org/10.1038/jhg.2016.52
15. Arbelo E, Protonotarios A, Gimeno JR et al. 2023 ESC guidelines for the management of cardiomyopathies: developed by the task force on the management of cardiomyopathies of the European Society of Cardiology (ESC). Eur Heart J 2023;44:3503–626. https://doi.org/10.1093/eurheartj/ehad194
16. Leggit JC, Whitaker D. Diagnosis and management of hypertrophic cardiomyopathy: updated guidelines from the ACC/AHA. Am Fam Physician 2022;105:207–09. Available from: https://www.aafp.org/pubs/afp/issues/2022/0200/p207.html
17. Arends M, Wanner C, Hughes D et al. Characterization of classical and nonclassical Fabry disease: a multicenter study. J Am Soc Nephrol 2017;28:1631–41. https://doi.org/10.1681/ASN.2016090964
18. El Sayed M, Hirsch A, Boekholdt M et al. Influence of sex and phenotype on cardiac outcomes in patients with Fabry disease. Heart 2021;107:1889–97. https://doi.org/10.1136/heartjnl-2020-317922
19. Nordin S, Kozor R, Bulluck H et al. Cardiac Fabry disease with late gadolinium enhancement is a chronic inflammatory cardiomyopathy. J Am Coll Cardiol 2016;68:1707–08. https://doi.org/10.1016/j.jacc.2016.07.741
20. Umer M, Kalra DK. Cardiac MRI in Fabry disease. Front Cardiovasc Med 2023;9:1075639. https://doi.org/10.3389/fcvm.2022.1075639
21. Pieroni M, Moon JC, Arbustini E et al. Cardiac involvement in Fabry disease. J Am Coll Cardiol 2021;77:922–36. https://doi.org/10.1016/j.jacc.2020.12.024
