Atrial fibrillation (AF) is a relatively common condition. The national prevalence for England on the latest quality and outcomes framework data is 1.3% and as many as 10% of patients aged over 75 may be in AF. On average, all of us have a 20–25% lifetime risk of developing AF.
An average GP will have 16–20 cases on their personal list and can expect to diagnose three new cases per annum. However, it is recognised that many cases of AF go undiagnosed and opportunistic screening for AF has been recommended. Of note, AF is responsible for 45% of embolic strokes. With the increasing emphasis on AF from Chapter 8 of the National Service Framework for Coronary Heart Disease (NSF-CHD), the National Stroke Strategy1 and inclusion of AF in the Quality and Outcomes Framework – as well as publication of the National Institute for Health and Clinical Excellence (NICE) guidelines for AF management – it is important for GPs to diagnose, treat and refer AF patients correctly.
There are three main areas to consider in AF patients: first, the diagnosis and treatment of any underlying co-morbid condition; second, symptom control by either a rate- or rhythm-control strategy; and third, the reduction of the accompanying risk of stroke and thromboembolism by appropriate prescription of antithrombotic therapy. Many straightforward cases of AF can be satisfactorily managed entirely in primary care, using the following structured approach.
1. Diagnose AF
Many patients with AF remain asymptomatic and undetected, and AF is usually suspected when a patient is found to have an irregular pulse. At fast or slow rates the irregularity can be hard to detect. Confirmation of a diagnosis of AF must be obtained by undertaking an electrocardiogram (ECG).2 Automatic reporting software is not very effective at diagnosing AF and can over diagnose when the baseline is indistinct or, alternatively, may miss cases. The characteristic ECG findings are irregularly irregular QRS complexes and the absence of consistent P waves. Practice nurses and GPs should take advantage of scenarios for opportunistic AF screening, e.g. at blood pressure (BP) checks or possibly flu clinics. Patients attending clinics for ischaemic heart disease, stroke, heart failure or diabetes should be systematically screened, as these conditions are commonly associated with AF.
2. Establish duration and type of AF
Based on a temporal classification, AF can be recent onset (within 48 hours), paroxysmal, persistent (i.e. duration of seven days or more, and continuing until terminated by drugs or cardioversion) or permanent (duration greater than one year or refractory to cardioversion attempts). This classification offers a simple approach and may help guide treatment objectives and management strategies. Obviously a first bout of AF may be either a paroxysm, persistent or permanent, and only further investigation will establish this.
If the AF is symptomatic and the patient can precisely pinpoint a recent onset (e.g. due to an obvious precipitant, such as alcohol binge or chest infection) then cardioversion is more likely to be effective, if no other adverse features are present. In many cases, however, the AF is asymptomatic and duration may be impossible to ascertain accurately. The longer the duration of AF, the less likely it is that cardioversion will be successful in restoring and maintaining sinus rhythm. AF of a duration greater than 12 months is unlikely to remit successfully with cardioversion. The overall success of DC cardioversion at one year is approximately 50%.3
3. Assess symptom severity
This can vary from patients in fast AF who are acutely breathless, dizzy and with rate-related chest discomfort who will need urgent hospital admission, to patients who appear to be asymptomatic. Some patients will report decreased exercise tolerance and generalised fatigability, but some elderly sedentary patients may even tolerate rates of 100 at rest with no reported symptoms. Assessing the impact of AF on the patient’s quality of life and taking account of other co-morbidities contributing to fatigue and dyspnoea will help in choosing a rate-control versus a rhythm-control strategy. Indeed, the sedentary elderly will often do well with a rate-control strategy, but younger, more active patients may well have better functional status and improved quality of life with a rhythm-control strategy. Mortality and long-term outcomes have been established to be no different between these strategies, for most patients.4 In patients aged over 65 with coronary artery disease, rate control has superior outcomes.4 A rhythm-control strategy is also preferable in those with lone AF or AF triggered by external factors.
Many AF patients fall in-between the categories of fit–young or sedentary–old, and the best treatment strategy for them will have to be individually determined, taking account of patient preference after a discussion of the pros and cons of each method. The Atrial Fibrillation Society (www.atrialfibrillation.org.uk) provides good-quality information for patients to aid in understanding treatment choices. There is an increasing move to rate control to avoid the side effects of rhythm-control drugs, which incur an increased risk of hospitalisations, based on trial data.5
4. Establish the cause
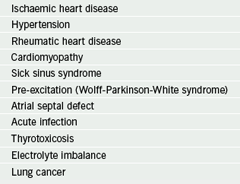
Common causes of AF (table 1) include ischaemic heart disease, hypertension, valvular heart disease, alcohol excess, cardiomyopathy and thyrotoxicosis. AF can be precipitated by many non-cardiac factors (e.g. infections – especially chest infections, electrolyte imbalance, or post surgery). History taking should include specific questioning to identify possible precipitants of AF.
5. Enquire about relevant co-morbidities
Any history of stroke, transient ischaemic attack (TIA) or amaurosis fugax will be highly relevant to decision making around anticoagulation therapy. It is also important to establish whether the patient has diabetes mellitus, coronary artery disease or congestive heart failure, as these disorders increase the risk of AF. Any history of gastrointestinal bleeding, ulceration or undiagnosed dyspepsia will be highly relevant to the use of anti-coagulation therapy, as will any history of bleeding tendencies or falls. An assessment of cognitive functioning is important to assess potential compliance with medication.
6. Undertake a physical examination of the patient
Measure the pulse rate measured at the apex, and measure the BP manually, automated measurement of BP in AF patients may be inaccurate. Examination should include auscultation for cardiac murmurs, and examination for signs of heart failure or thyrotoxicosis.
7. Undertake the following tests
Check the full blood count and clotting screen, urea and electrolytes, liver function tests, thyroid function tests, glucose and cholesterol levels. Enclose a copy of the ECG with the referral. Echocardiography is helpful in the management of AF to diagnose structural heart disease or left ventricular systolic dysfunction, but is not indicated for all cases as it may have little effect on management. According to local access arrangements, it may be helpful to undertake echocardiography prior to referral in selected cases, as this may speed up treatment decisions, for example, around cardioversion. Echocardiography can aid in refinement of stroke risk stratification and help predict the likelihood of successful cardioversion.
8. Reduce symptoms by prescribing rate-controlling medication
For patients who are symptomatic at presentation, rate-control medication should be initially commenced. For most, a beta blocker or rate-limiting calcium channel blocker (e.g. diltiazem or verapamil) would offer good rate control. For the sedentary–elderly subject, digoxin can be effective, started with a loading dose of 250–500 µg (or lower if renal function is impaired) and continuing with a maintenance dose of 125 µg.
When initiating a beta blocker, a small dose should be commenced and titrated up to control the apical rate at 80 at rest and 140 on mild-to-moderate exertion. In assessing rate control, the rate should always be assessed by auscultation at the apex rather than palpation of the radial pulse. Rate control on exertion can easily be assessed by asking the patient to walk 100–200 yards around the surgery. Effective rate control prior to referral also helps in the acquisition of good quality images if the patient undergoes echocardiography.
9. Start the patient on appropriate anticoagulation
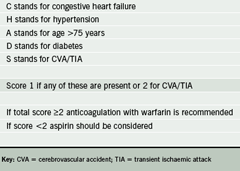
Appropriate antithrombotic therapy should be started without delay regardless of the type of AF or whether a rate or rhythm strategy is ultimately to be applied. The risk of thromboembolic events is high immediately after the onset of AF. The GP should give a full explanation of the risks and benefits of anticoagulation to the patient in terms that are easily understood. NICE has produced a detailed algorithm for deciding on appropriate antithrombotic therapy, but the CHADS2 score is simpler and easy to use (table 2).6
Warfarin confers a 68% reduction in stroke risk, whereas the risk reduction with aspirin is a modest 22%.7 The latter is likely to be the effect of aspirin on vascular disease or cardiovascular risk factors, rather than the effect related to AF per se. Aspirin can be considered as a second best when a patient is unable to comply with the variable-dose regimen of warfarin and the need for monitoring. If anticoagulation is contraindicated as a result of unacceptable risk of haemorrhage, or declined by the patient, then this should be recorded with the appropriate code for Quality Outcomes Framework purposes.
10. Carefully consider the reason for referral
Clearly state the reasons for referral in the referral letter. Elderly, mildly symptomatic patients suitable for a rate-control strategy can be appropriately managed in primary care. Decisions around anticoagulation are often best left with the GP who will have the most comprehensive knowledge of the patient’s medical history and personal circumstances.
Reasons for referral include diagnosis of underlying structural heart disease, difficulties achieving adequate rate control, advice on choice of rhythm control (medication, peri-cardioversion, etc.), paroxysmal AF (a difficult condition!) and consideration for DC cardioversion. Most cases of AF are suitable for referral to a general cardiologist.
The expertise of an electrophysiologist is only needed for patients with recurrent atrial flutter, Wolff-Parkinson-White syndrome, and patients with symptomatic AF despite optimal drug therapy, as these patients may be candidates for ablation procedures.
Conflict of Interest
RH has received horaria and travel bursaries from Astra Zeneca, Solvay, Bristol-Myers Squibb, Pfizer, Novartis and Merck Sharp & Dohme. GYHL: none declared.
Editors’ note
An editorial on the use of electrical cardioversion for atrial fibrillation can be found on pages 281–2 of this issue.
References
- Department of Health. National stroke strategy. London: Department of Health publications, 284536 1p8k Dec 07.
- Royal College of Physicians. Atrial fibrillation, national clinical guideline for management in primary and secondary care. London: Royal College of Physicians, 2006.
- Lip GY, Tse HF. Management of atrial fibrillation. Lancet 2007;370:604–18.
- Wyse DG, Waldo AL, Di Marco JP et al. A comparison of rate versus rhythm control in patients with AF. N Engl J Med 2002;347:1825–33.
- Carlsson J, Miketic S, Windeler J et al. Randomized trial of rate control versus rhythm control in persistent AF. The Strategy of Treatment of AF (STAF) Study. J Am Coll Cardiol 2003;41:1690–6.
- Gage BF, Waterman AD, Shannon W et al. Validation of clinical classification scheme for predicting stroke – results from the National Registry of AF. JAMA 2001;285:2864–7.
- Lip GY, Lim HS. Atrial fibrillation and stroke prevention. Lancet Neurol 2007;6:981–93.
