Microscopic polyangiitis is a systematic necrotising vasculitis that affects small vessels without granulomata. Typically the most common manifestation is renal involvement. We report an unusual presentation of microscopic polyangiitis in a young male.
Case report
A 19-year-old white male non-smoker without any significant past medical, travel or drug history presented to Accident & Emergency with a one-week history of dry cough, muscle aches, night sweats, and left-sided pleuritic chest pain. On admission his temperature was 38.4°C, pulse 118 beats per minute and blood pressure 130/70 mmHg. The jugular venous pressure was elevated 5 cm above the sternal angle. He had normal heart sounds with a pericardial rub. There were no other signs and urine testing was normal.
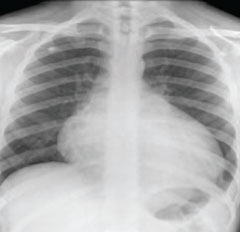
His inflammatory markers were raised with C-reactive protein (CRP) of 174 mg/L (normal range [NR] <5 mg/L), erythrocyte sedimentation rate (ESR) of 76 mm/h (NR 0–10 mm/h) and white cell count (WCC) of 10.0 g/L. Kidney and liver function were normal. The electrocardiogram (ECG) showed sinus tachycardia. The chest radiograph showed cardiomegaly (figure 1). The echocardiogram showed a small circumferential pericardial effusion with no other abnormalities. Computed tomography (CT) scan of the chest confirmed a small pericardial effusion.
He was started on broad-spectrum antibiotics intravenously, although blood, sputum and urine cultures subsequently returned with no growth. Over the next four days his pyrexia persisted and a diagnostic pericardiocentesis was performed: 100 ml of blood-stained pericardial fluid was sent for examination revealing protein 49 g/L and glucose 2.9 mmol/L. Cytology revealed blood, abundant acute inflammatory cells, macrophages and fibrinous exudates with no malignant cells. The report suggested an autoimmune condition such as systemic lupus erythromatosus (SLE). No bacterial growth was present (including tuberculosis cultures). With intravenous antibiotics his fever, sweating and elevated inflammatory markers improved and after two weeks antibiotics were stopped.
Human immunodeficiency virus (HIV) and hepatitis A, B, C, and autoimmune profile (antinuclear antibody, antimitochondrial antibody, antismooth muscle antibody and antiparietal cells antibody) were all negative. C3 and C4 complement levels and 24-hour urinary clearance studies were also normal.
He was re-admitted six weeks after the initial presentation, unwell and complaining of the sharp central chest pain similar to his first admission. His temperature was 39.8°C, CRP 101 mg/L and ESR 50 mm/h with normal WCC and kidney function tests. Physical examination was within normal limits. The echocardiogram showed a 1 cm global pericardial effusion. On admission we received his antineutrophil cytoplasmic antibodies (ANCA) results: the pANCA was strongly positive with myeloperoxidase-ANCA being greater than 100 U/ml (NR 0–6 U/ml) and cANCA normal at 1 U/ml (NR 0–6 U/ml). A diagnosis was therefore made of ANCA-positive microscopic polyangiitis (MPA) and he was pulsed with 1 g methylprednisolone intravenously. His symptoms improved dramatically with normalisation of CRP and ESR within less than a week. He was discharged home with out-patient follow-up in the rheumatology clinic.
One year later he is asymptomatic on low-dose prednisolone and mycophenolate mofetil.
Discussion
This 19-year-old man presented acutely with a vasculitic illness causing constitutional symptoms and pericarditis. Subsequent serology confirmed a diagnosis of MPA. Despite a short delay in diagnosis he has done well with low-dose steroid treatment and mycophenolate mofetil.

The vasculitides are categorised by the size (small, medium and large) of the vessel affected as shown in figure 2.1,2
MPA is defined as systematic necrotising vasculitis that clinically and histologically affects small vessels without granulomata.3 In the UK the incidence is less than two in 100,000, with an equal sex distribution and an average age of onset in the fifth decade.2 Clinical features include constitutional symptoms such as fever, anorexia, fatigue and weight loss. Typically, renal involvement is the most common manifestation (79% patients).4
Cardiac involvement is uncommon and usually occurs in the context of multi-system involvement. In the largest series of MPA, 85 patients were studied; only 10% had pericarditis, with which our patient presented, other cardiac conditions included cardiac failure occurring in 18% and myocardial infarction in 2%.4 We have not found any case reports of pericardial effusion in MPA.
Our patient is also unusual in that his presentation was acute. There is often a long time lapse between the onset of symptoms and diagnosis of MPA.4
Although our patient is well one year after presentation, his follow-up will need to be prolonged. The prognosis of MPA is variable and the condition may follow a relapsing course. In the largest reported retrospective series, after a mean follow-up of 70 months, 28 of the 85 (33%) patients with MPA had died, 29 patients (34%) relapsed after a mean follow-up of 43 months and a further five had a second relapse.4 Renal involvement is a poor prognostic factor and increases the frequency of relapses.3
The cornerstone of treatment is corticosteroids but immunosuppressive therapy with mycophenolate mofetil or cyclophosphamide may be used as a steroid-sparing agent. All patients who present with an unexplained pericarditis or a pericardial effusion should be specifically tested for ANCA.
Conflict of interest
None declared.
References
- Mansi I, Opran A, Rosner F. ANCA-associated small-vessel vasculitis. Am Fam Physician 2002;65:1615–20.
- Jennette JC, Falk RJ. Small-vessel vasculitis. N Engl J Med 1997;337:1512–23.
- Lhote F, Cohen P, Genereau T, Gayraud M, Guillevin L. Microscopic polyangiitis: clinical aspects and treatment. Ann Med Interne (Paris) 1996;147:165–77.
- Guillevin L, Durand-Gasselin B, Cavellos R et al. Microscopic polyangiitis: clinical and laboratory findings in eighty five patients. Arthritis Rheum 1999;42:421–30.
