Hypertension is a growing clinical burden associated with significant morbidity and mortality. Those patients who remain with uncontrolled blood pressure despite multiple appropriate tablets are labelled as resistant hypertension. This cohort faces the highest risk. A key driving factor in resistant hypertension is an abnormally elevated sympathetic nervous system (SNS). It is now possible to attenuate this non-pharmacologically by performing radiofrequency ablation to the renal sympathetic nerves using a transcatheter approach. Currently available trial data show impressive blood pressure reductions with this therapy and, more importantly, its relative safety. The National Health Service (NHS) experience with this procedure is at an early stage, but is likely to grow with guidance already published by the joint British Societies and National Institute for Health and Care Excellence (NICE).
Introduction
 Hypertension is an epidemic of our time. It is strongly associated with hard end points such as acute coronary syndromes, heart failure, stroke, renal failure and death. It is anticipated that by 2025, 29% of the world population (over 1 billion individuals) will have this condition.1 In the UK, approximately 18 million adults suffer from hypertension, of which only 10 million are on blood-pressure-lowering treatment.2 Amongst patients who are aware of their condition and are prescibed antihypertensive drugs, only 50% achieve adequate control, due to insufficient drug treatment or poor compliance.2 More rarely, patients, who regularly use three or more antihypertensives, including a diuretic, persistently have systolic blood pressures of greater than 140 mmHg or diastolic blood pressures greater than 90 mmHg and meet the criteria for resistant hypertension. It is important to identify this group because they have almost double the cardiovascular mortality, which, with appropriate specialist input, can be reduced by controlling their blood pressure.3 This specialist input covers dietary and lifestyle modifications, exclusion of secondary hypertension, alternative pharmacological intervention and novel invasive interventions. Renal artery sympathetic nerve denervation (RDN) is one such non-pharmacological therapy that is available in the hypertension specialist’s armoury to manage resistant hypertension.
Hypertension is an epidemic of our time. It is strongly associated with hard end points such as acute coronary syndromes, heart failure, stroke, renal failure and death. It is anticipated that by 2025, 29% of the world population (over 1 billion individuals) will have this condition.1 In the UK, approximately 18 million adults suffer from hypertension, of which only 10 million are on blood-pressure-lowering treatment.2 Amongst patients who are aware of their condition and are prescibed antihypertensive drugs, only 50% achieve adequate control, due to insufficient drug treatment or poor compliance.2 More rarely, patients, who regularly use three or more antihypertensives, including a diuretic, persistently have systolic blood pressures of greater than 140 mmHg or diastolic blood pressures greater than 90 mmHg and meet the criteria for resistant hypertension. It is important to identify this group because they have almost double the cardiovascular mortality, which, with appropriate specialist input, can be reduced by controlling their blood pressure.3 This specialist input covers dietary and lifestyle modifications, exclusion of secondary hypertension, alternative pharmacological intervention and novel invasive interventions. Renal artery sympathetic nerve denervation (RDN) is one such non-pharmacological therapy that is available in the hypertension specialist’s armoury to manage resistant hypertension.
History
Texts dating back to ancient China (2600 BC) have commented on ‘hard pulse disease’ and its association with stroke. In those times, treatment for this condition was limited to acupuncture, venesection and leeches.4 Not much clinical progress was made until techniques to measure blood pressure were developed. Reverend Hales was the first to measure it in 1733. He directly cannulated a horse’s left crural artery with some brass tubing and, when he connected it to a glass column, the blood rose vertically by 8 feet and 3 inches. Building on this work, and that of others, Rocci, in 1896, developed the early mercury sphygmomanometer that with manual palpation of the distal pulse could estimate the systolic blood pressure. Then in 1904, Korotkoff described the sounds heard with a stethoscope that could be used to accurately assess, not only systolic, but diastolic blood pressure.4
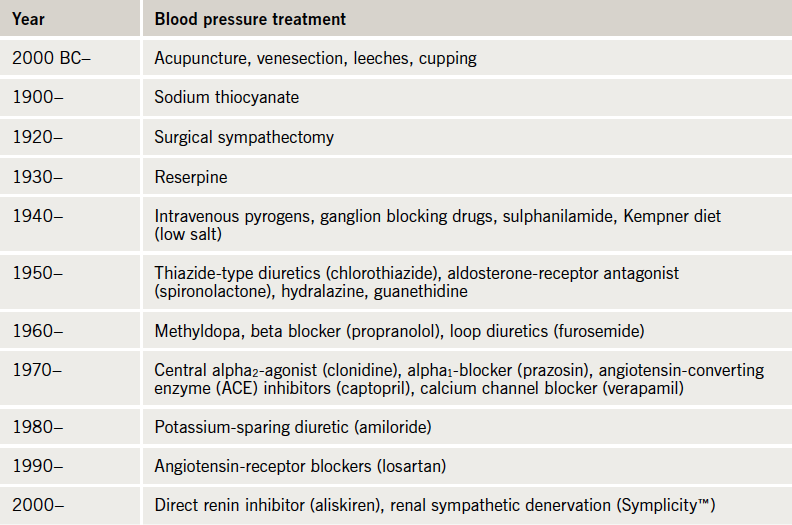
In the early 1900s, the most effective treatments available for hypertension were salt restriction and surgical non-selective sympathectomy, both relatively effective but limited by tolerability. Oral medications had not been established. One compound tried in that time was a cyanide derivative and, not surprisingly, its popularity was short-lived. It was not until 1957 that a safe orally effective antihypertensive, a diuretic (chlorothiazide) was available.4 Since then, a number of different classes have been established, the historical time frame has been summarised in table 1. However, these drugs are not effective in everyone and between 2 and 10% of our known hypertensive patients remain resistant to treatment.
Drugs do not always work
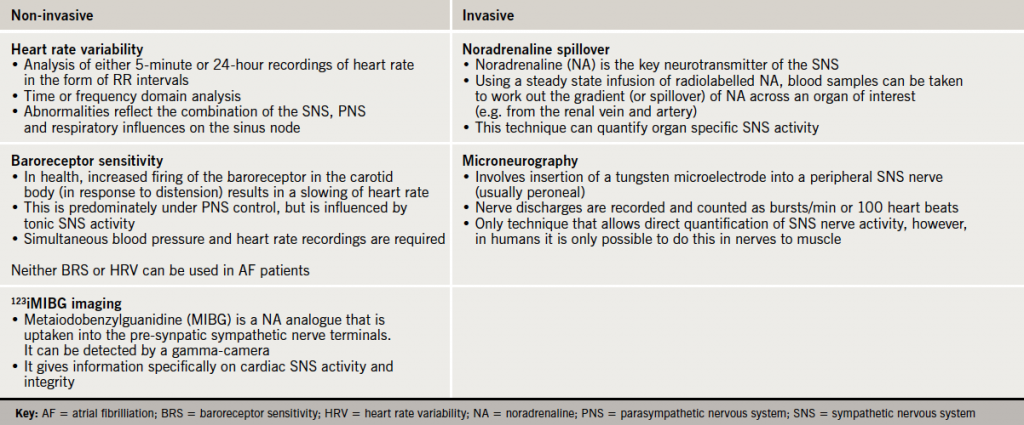
Poor compliance and intolerable side effects are key causes of poor blood pressure control. Patients with true resistant hypertension commonly have a persistently heightened sympathetic nervous system (SNS).5,6 There is no single gold-standard measure of sympathetic activation, and each of the techniques available give different information about the nature of its activation (table 2). Human data using all of these techniques confirm that the SNS is activated, not only in essential hypertension, but also in pregnancy-related, white-coat, and in some secondary causes of hypertension. Furthermore, the more advanced the degree of hypertension the greater the degree of sympathetic activation.5
In particular, as shown using animal models, the SNS activity to and from the kidney is abnormal in hypertension.7 Stimulating the renal efferent nerves (running from the central nervous system to the kidney) promotes noradrenaline (norepinephrine) and renin release, and causes increased sodium and water retention and renal vasoconstriction. Stimulation of the afferent nerves (running from the kidney to the central nervous system) perpetuates central nervous system sympathetic outflow across multiple organ beds. Interrupting the SNS efferents and afferents at the renal level abolishes these reflexes to stimulation. Along with this work, there is a much wider literature, which suggests that an overactive SNS, not only sustains hypertension, but also initiates it.5,7
Currently available medications, including beta blockers, renin-angiotensin-aldosterone system blockers and monoxidine either directly or indirectly attenuate the SNS. However, these drugs do not completely attenuate the excess activation that is characteristic in hypertension. Furthermore, some drugs can directly cause iatrogenic sympathetic activation. A study of hypertensive patients showed that a combination of an angiotensin-receptor blocker and a diuretic increased sympathetic activity, as measured by muscle sympathetic nerve activity, despite achieving good blood pressure control.8
Surgical removal of the renal sympathetic nerves and the effect on blood pressure
Renal transplant medicine has given us much insight into the safety and long-term effects of renal sympathectomy. First, there is much reassurance in the fact that donor kidneys are able to function normally in transplant recipients, despite the fact that they do not have functioning efferent and afferent sympathetic nerves. Second, when there is uncontrolled hypertension in patients with well-functioning renal allografts, performing native kidney nephrectomy (which also includes a renal sympathectomy), not only reduces central sympathetic outflow (as demonstrated by a reduced MSNA or muscle sympathetic nerve activity), but also controls blood pressure, regresses left ventricular hypertrophy and improves allograft blood flow.9
In the early 20th century, when no suitable blood pressure reduction tablets were available, malignant hypertension (a condition characterised by excessive blood pressure and end organ damage, e.g. papilloedema) had almost 100% five-year mortality. Consequently, both patients and physicians were keen to trial any treatment that could potentially improve this dismal prognosis. It was in this climate that Breuning is reported to have performed the first surgical sympathectomy for hypertension in 1923. This procedure was non-selective and, hence, affected the SNS innervations of several organ beds, not just the kidneys. This often caused a multitude of debilitating side effects. Its effect on blood pressure was encouraging but variable and, hence, once oral therapies were established, this procedure fell out of favour.
Renal sympathetic denervation
Drawing on the previous decades of accumulated knowledge on the SNS and its harmful involvement in hypertension, it was a logical extension to develop techniques to selectively attenuate it at the renal level using a minimally invasive method.
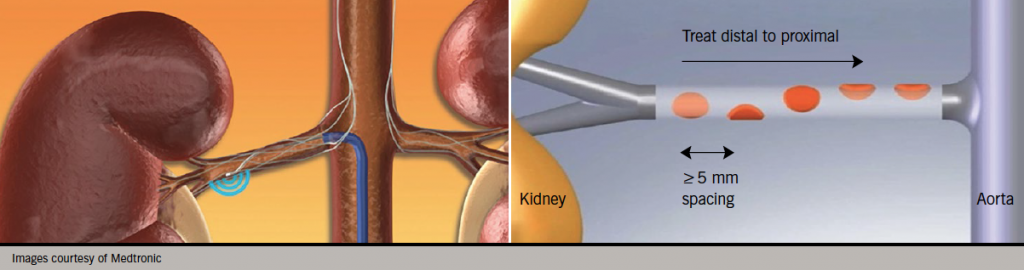
Anatomically, the renal afferent and efferent sympathetic nerves run together in the adventitia of the renal artery. The majority of the nerves are found within 3 mm of the artery lumen. This relation makes them accessible to potential therapies that could be delivered from within the renal artery lumen. There are now several different devices that can be used to target these nerves transluminally, all coming under the general denomination of renal sympathetic denervation or RDN. It involves passing a floppy ablation catheter into the renal artery using conventional angiography techniques. The ablation catheter is positioned using fluoroscopy and therapy (most commonly radiofrequency or RF) is delivered using an automated generator which has pre-programmed safety algorithms in a helical pattern from distal to proximal (figure 1). The helical pattern was chosen first as histology of human renal arteries showed that the sympathetic nerves are circumferentially located around each artery and arborise from proximal to distal. Thus, to achieve a greater degree of sympathetic nerve block, RDN would have to be performed at multiple locations. Second, to minimise the risk of inducing renal artery stenosis, a helical pattern of ablation as opposed to circular was adopted. Normally, four to six sites per artery are ablated.
The trials
After many animal studies, which have involved rats, pigs and dogs, with and without hypertension, the technique was first translated to human use in 2007, using the Symplicity™ catheter, first developed by Ardian Inc., USA, subsequently acquired by Medtronic Inc., USA.10 The reported data for 64 out of the 153 patients denervated in the original open-label Symplicity HTN-1 study showed an average blood pressure reduction of 23/11 mmHg at 12 months. This reduction was sustained in the subset of 18 patients who had completed 24 months of follow-up, which reassured investigators who had seen some evidence of nerve re-innervation in rat models. Symplicity HTN-2 was the first randomised-controlled trial of RDN.11 It recruited 106 patients and demonstrated a blood pressure reduction of 32/12 mmHg with denervation. Other groups have confirmed these findings, but in studies involving similar small numbers. The currently published trials have shortcomings, which affect their interpretation. Notably, the patients were not blinded, office blood pressure rather than 24-hour ambulatory blood pressure was used to assess response, and less than a quarter of patients were on aldosterone-receptor antagonists.
Symplicity HTN-3 is currently ongoing in the USA and has addressed some of these shortcomings. It aims to enrol 530 patients. The trial design is randomised with single blinding. The primary end point is change in office blood pressure at six months with the secondary end point of change in 24-hour ambulatory blood pressure. It is anticipated that the trial will complete in 2014.
Even though blood pressure per se is a good surrogate end point, to cast aside any doubt of RDN’s value in hypertension, there needs to be an appropriately powered hard end point trial, which currently has not been planned. At least 10% of patients who receive RDN show no response (reduction <10 mmHg) and, unsatisfactorily, the reasons for this are unclear.
Patient selection
Renal sympathetic denervation is not a panacea for hypertension or even resistant hypertension. The European Society of Cardiology (ESC), joint UK societies and National Institute for Health and Care Excellence (NICE) have stressed the importance of patient selection, which must be done in a multi-disciplinary format intimately involving a hypertension specialist. To be eligible for RDN, using NICE criteria, the patient must have at least stage 2 hypertension with an office systolic reading of >160 mmHg or an ambulatory daytime reading >150 mmHg. All patients should have progressed through the medications recommended at step 4 in the NICE/British Hypertension Society treatment algorithm, which includes angiotensin-converting enzyme (ACE) inhibitors/angiotensin-receptor blockers, calcium channel blockers, diuretics and one of spironolactone, higher-dose diuretic, beta blocker or alpha blocker. Lifestyle interventions must have been performed and compliance confirmed.
White-coat hypertension must be excluded with an ambulatory monitor. In a cohort of 8,295 resistant hypertension patients based on office readings only, 3,113 (37.5%) were reclassified as white coat (average readings of <130/80 mmHg) after ambulatory monitoring.12
Sympathetic nerve activity in primary hyperaldosteronism and renovascular disease is not frequently heightened and is similar to age-matched normotensive controls. Furthermore, there are effective treatments for other secondary causes and, hence, these patients are currently not eligible for RDN.
Prior to undertaking the procedure it is advisable to image the renal arteries according to local expertise to assess:
1. Size: the renal arteries ideally have to be greater than 4 mm in diameter to accommodate the ablation catheter.
2. Number: it would be important to identify multiple renal arteries as this would help plan the procedure.
3. Length: to achieve adequate ablation, a length of 20 mm of artery before any major bifurcation is necessary.
4. Structure: significant ostial/proximal stenosis or fibromuscular dysplasia would preclude renal denervation.
Current European guidelines recommend that RDN should be performed only in patients with an estimated glomerular filtration (eGFR) rate of >45 ml/min/1.73m2. From the Symplicity HTN-1 and -2 cohorts, no patient deteriorated to requiring dialysis support, though there were some patients that did have a >25% reduction in renal function at six months. The numbers of patients reported with long-term follow-up data with respect to renal function are low. Mahfoud et al. reported that in 88 patients who underwent RDN, no significant change in cystatin C GFR was observed.13 Studies have reported the use of this procedure in patients with eGFR of 30 ml/min/1.73m2, and even in those with end-stage renal failure. However, outside of a research setting or a clinical setting where there is close involvement of nephrologists, RDN should not be offered to those with eGFR <45 ml/min/1.73m2.
The devices
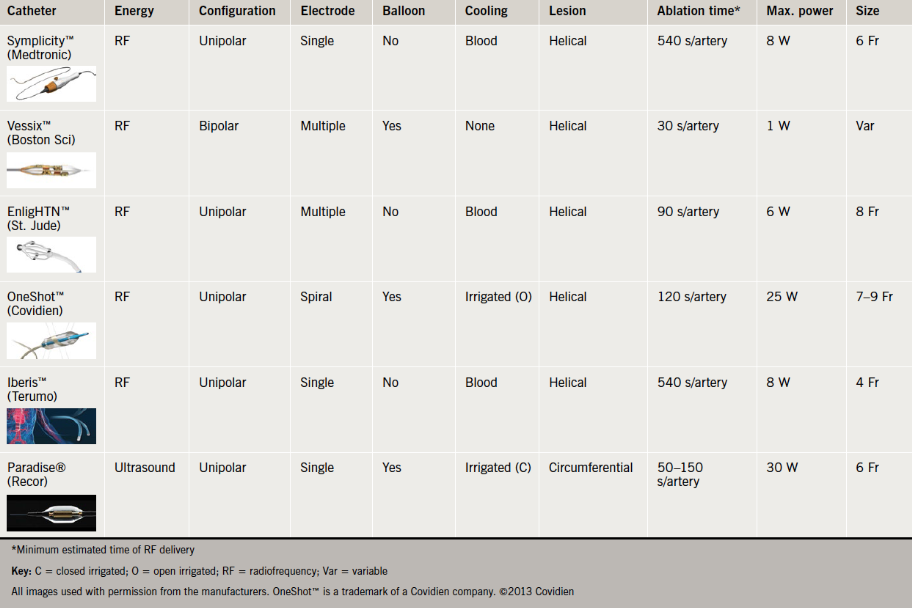
The Symplicity™ RDN catheter was the first device to be launched and, hence, has the most backing data. Other available catheters that also have a CE mark include EnligHTN™ (St. Jude Medical Inc., USA), V denervation system (Vessix Vascular Inc. now acquired by Boston Scientific, USA), OneShot™ (Maya Medical Inc. now acquired by Covidien, USA), Iberis™(Terumo, China) and Paradise® (Recor Medical Inc., USA). The Recor system uses ultrasound energy in a circumferential application rather than RF energy in a helical application (table 3). The Iberis™ catheter can be used via the radial artery as it is 4 Fr in size and comes in a longer length.
Other products in development include the Bullfrog®, which injects the neurotoxin, guanethidine, directly into the renal artery adventitia, and Kona’s ultrasound machine, which is used externally and, hence, is non-invasive.
Peri-procedural care
The procedure should be undertaken in an angiography laboratory with appropriate haemodynamic monitoring. The procedure is associated with abdominal and back pain related to the RF energy stimulation of C pain fibres, which are in close proximity to the renal sympathetic nerves. This mandates the need for appropriate analgesia, sedation and, if necessary, general anaesthesia. Currently, most centres in the UK keep the patient in overnight post-procedure.
Optical coherence tomography has shown that thrombus can occur in patients during RDN, which raises theoretical concerns of the risk of renal infarction due to distal embolisation.14 It is not known whether heparin and antiplatelet therapy reduces the thrombus burden associated with the procedure, but it is advised that they are administered.
Commissioning and funding
Currently, funding for the procedure is obtained on a named-patient basis from the relevant clinical commissioning group. There are plans to formally commission this service for the NHS via the Specialised Complex Invasive Cardiology Services group. In 2013–14, the procedure will be funded across selected centres in the UK who have the expertise and facilities to deliver the treatment to the carefully selected patients. It is anticipated that there will be a limited number of treatable candidates per centre and, hence, the necessity of a multi-disciplinary team to prioritise those patients that are most likely to benefit from it.
The future
Though very promising initial results have been achieved with renal denervation, there is yet a lot more work that needs to be undertaken before its role in the management of hypertension is chiselled in stone. First, we need blinded, randomised studies that use ambulatory blood pressure readings to enrol and assess efficacy of intervention. Second, there needs to be a study, powered to assess impact of therapy on mortality and long-term blood pressure control. Third, equivalence needs to be shown with all the different available RDN catheters.
Furthermore, there are now other autonomic nervous system modulating devices at various stages of development that could be exploited in hypertension management, e.g. baroreceptor stimulators. What is clear is that over the next few years our experience with this technology and how to wield it will increase, especially as it is currently being extended to heart failure, renal failure, metabolic syndrome and obstructive sleep apnoea.
Finally, this global resurgence of interest in blood pressure management is very welcome and, hopefully, will improve, not only management of this deadly condition, but also identification and patient education•
Declaration of support
HP is supported by the National Institute of Health Research (NIHR) funded Biomedical Research Unit at the Royal Brompton Hospital.
Conflict of interest
CDM has received speaker’s fees from Medtronic Inc.
HP: none declared.
Editors’ note
An editorial about renal denervation by Dr Melvin Lobo can be found on pages 128–9 of this issue.
Key messages
- Renal denervation therapy is National Institute for Health and Care Excellence (NICE) approved for patients with resistant hypertension
- Resistant hypertension is classified as blood pressure of >140/90 mmHg, despite being compliant with treatment that included at least three antihypertensive medications (one of which is a diuretic)
- The decision to undertake renal denervation must be made in a multi-disciplinary team, which must involve a hypertension specialist
- In suitable patients, this procedure is effective in lowering blood pressure with an acceptable risk profile
References
1. Kearney P, Whelton M, Reynolds K et al. Global burden of hypertension: analysis of worldwide data. Lancet 2005;365:217–23. http://dx.doi.org/10.1016/S0140-6736(05)17741-1
2. Caulfield M, de Belder M, Cleveland T et al. The joint UK societies’ consensus statement on renal denervation for resistant hypertension. London: Joint UK Societies, January 2012. Available from: http://www.bhsoc.org/docs/The-Joint-UK-Societies%27-Consensus-on-Renal-Denervation-for-resistant-hypertension.pdf
3. Doumas M, Papademetriou V, Douma S et al. Benefits from treatment and control of patients with resistant hypertension. Int J Hypertension 2011; Article ID 318549. http://dx.doi.org/10.4061/2011/318549
4. Esunge P. From blood pressure to hypertension: the history of research. J R Soc Med 1991;84:621. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1295564/
5. Mancia G, Grassi G, Giannattasio C, Seravalle G. Sympathetic activation in the pathogenesis of hypertension and progression of organ damage. Hypertension 1999;34:724–8. http://dx.doi.org/10.1161/01.HYP.34.4.724
6. Tsioufis C, Kordalis A, Flessas D et al. Pathophysiology of resistant hypertension: the role of sympathetic nervous system. Int J Hypertens 2011; Article ID 642416. http://dx.doi.org/10.4061/2011/642416
7. Di Bona G, Esler M. Translation medicine: the antihypertensive effect of renal denervation. Am J Physiol Regul Intergr Comp Physiol 2010;298:R245–R253. http://dx.doi.org/10.1152/ajpregu.00647.2009
8. Fu Q, Zhang R, Witkowski S et al. Persistent sympathetic activation during chronic antihypertensive therapy: a potential mechanism for long term morbidity? Hypertension 2005;45:513–21. http://dx.doi.org/10.1161/01.HYP.0000158312.63381.c1
9. Getts R, Hazlett S, Sharma S et al. Regression of left ventricular hypertrophy after bilateral nephrectomy. Nephrol Dial Transplant 2006;21:1089–91. http://dx.doi.org/10.1093/ndt/gfi321
10. Krum H, Schlaich M, Whitbourn R et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 2009;373:1275–81. http://dx.doi.org/10.1016/S0140-6736(09)60566-3
11. Symplicity HTN-2 Investigators. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet 2010;376:1903–09. http://dx.doi.org/10.1016/S0140-6736(10)62039-9
12. De la Sierra A, Segura J, Banegas J et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension 2011;57:898–902. http://dx.doi.org/10.1161/HYPERTENSIONAHA.110.168948
13. Mahfoud F, Cremers B, Janker J et al. Renal hemodynamics and renal function after catheter-based renal sympathetic denervation in patients with resistant hypertension. Hypertension 2012;60:419–24. http://dx.doi.org/10.1161/HYPERTENSIONAHA.112.193870
14. Templin C, Jaguszewski M, Ghadri J et al. Vascular lesions induced by renal nerve ablation as assessed by optical coherence tomography: pre- and post-procedure comparison with the Symplicity catheter system and the EnlighTHN multi-electrode renal denervation catheter. Euro Heart J 2013;34:2141–8. http://dx.doi.org/10.1093/eurheartj/eht141
