A new emphasis on estimating the lifetime risk of future cardiovascular disease (CVD) – rather than the current 10-year risk – has been recommended by the Joint British Societies in their report Consensus Recommendations for the Prevention of Cardiovascular Disease (JBS3). The new recommendations will encompass much more of the population, such as young individuals and women, who may have a high lifetime risk of CVD but a low 10-year risk using current guidance.
This emphasis on short-term risk means the long-term consequences of modifiable risk factors may be overlooked. Age and male gender are currently the biggest drivers of risk, said Professor John Deanfield (Chair of JBS3 and British Heart Foundation Chair of Cardiology at UCL, London) at a launch event for the JBS3 recommendations. Treatment has favoured elderly men, with risk calculators disenfranchising the young, especially women, he added.
The report, which the British Cardiovascular Society calls “an evolution in CVD prevention,” supports intensive management of people at high lifetime risk for CVD. Its message that early intervention will give long-term dividends is one that the population can understand, said Professor Deanfield.
A key feature of JBS3 is a novel online risk calculator, which communicates clearly to individuals their long-term risk of CVD, using tools such as a person’s ‘heart age’ and years of CVD event-free survival (see figure 1). Healthcare professionals can then show in different graphical formats how lifestyle modifications and other interventions, such as drug treatment when appropriate, can increase a patient’s years of healthier life.
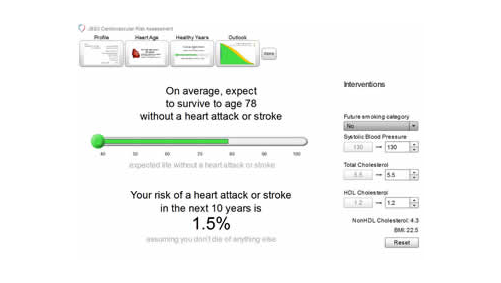
It is hoped that this personalised approach will help empower people to invest in their future cardiovascular health. Despite improvements in CVD over the past 20 years in the UK, it is still a major cause of morbidity and mortality, resulting in over 160,000 deaths a year.
Both the full guidance and risk calculator are available free of charge. The guidance can be downloaded from Heart (http://www.heart.bmj.com) and the risk calculator can be downloaded at http://www.jbs3risk.com. The Joint British Societies comprise: British Cardiovascular Society, Association of British Clinical Diabetologists, British Association for Cardiovascular Prevention and Rehabilitation, British Association for Nursing in Cardiovascular Care, British Heart Foundation, British Hypertension Society, British Renal Soceity, Diabetes UK, HEART UK – The Cholesterol Charity, Renal Association and the Stroke Association.
New treatments for PAH and CTEPH available
A new class of drug, the soluble guanylate cyclase (sGC) stimlator riociguat (Adempas®, Bayer), has been approved by the European Medicines Agency (EMA) for the treatment of two forms of pulmonary hypertension: chronic thromboembolic pulmonary hypertension (CTEPH) and pulmonary arterial hypertension (PAH). Actelion Pharmaceuticals has also announced the availability in England of its novel endothelin receptor antagonist, macitentan (Opsumit®) as a new treatment option for patients with PAH.
Both PAH and CTEPH are rare conditions but can have a devastating impact on the lives of patients.
Riociguat
Riociguat is the first drug to be approved for the treatment of CTEPH. There are an estimated 1,300 sufferers of CTEPH in the UK. The condition is normally treated surgically by pulmonary endarterectomy but many patients (20–40%) are inoperable, and the condition can recur or persist in some patients.
Results from the CHEST-1 (Chronic Thromboembolic Pulmonary Hypertension Soluble Guanylate Cyclase-Stimulator Trial 1) study showed that in patients with inoperable CTEPH or recurrent or persistent disease after surgery, riociguat significantly improved their exercise capacity, with patients in the riociguat group able to walk an average of 46 additional metres (95% CI [25–67 m], p < 0.0001) in the six-minute walk test (6MWT) from baseline after 16 weeks compared with placebo.
In PAH, results from the PATENT-1 (Pulmonary Arterial Hypertension sGC-Stimulator Trial) study showed patients treated with riociguat were able to walk an average of 36 additional metres (95% CI [20–52 m], p<0.0001) in the 6MWT compared with patients on placebo.
Riociguat acts on sGC, an enzyme which acts as the receptor for nitric oxide. By stimulating sGC, riociguat enhances synthesis of the cyclic guanosine monophosphate (cGMP) which plays an important role in regulating vascular tone, proliferation, fibrosis and inflammation.
Macitentan
Macitentan is indicated for use as monotherapy, or in combination, for the long-term treatment of PAH in adult patients of WHO Functional Class (FC) II to III. England is one of the first countries in Europe to make macitentan available to prescribe, following EU marketing authorisation based on data from the phase III study, SERAPHIN (Study with an Endothelin Receptor Antagonist in Pulmonary Arterial Hypertension to Improve Clinical Outcome).
This showed that treatment with macitentan 10 mg resulted in a 45% relative risk reduction (RRR) (hazard ratio [HR] 0.55; 97.5% CI: 0.39–0.76; p < 0.001) of the composite morbidity-mortality end point when compared to placebo. In patients already on background therapy for PAH, treatment with macitentan 10 mg resulted in a 38% RRR (HR 0.62; 95% CI: 0.43–0.89; p=0.009) of the composite morbidity-mortality endpoint compared to placebo.
Macitentan also significantly reduced the risk of PAH-related death or hospitalisation up to end of treatment and improved quality of life. Its efficacy has been shown in a PAH population including idiopathic and inherited PAH, PAH associated with connective tissue disorders, and PAH associated with corrected simple congenital heart disease. Efficacy was also demonstrated in treatment naïve patients and those already receiving PAH-specific background therapies, such as phosphodiesterase type 5 inhibitors
