National Institute for Health and Care Excellence (NICE) guidance supports the introduction of sacubitril/valsartan under the supervision of a heart failure specialist with access to a multi-disciplinary heart failure team. Clinical information was obtained retrospectively on all patients with a primary coded diagnosis of heart failure discharged from the Conquest Hospital, Hastings, UK during the calendar year 2015. We recorded the proportion of patients meeting the NICE recommendation and those patients meeting the additional PARADIGM-HF (Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure) study inclusion criteria.
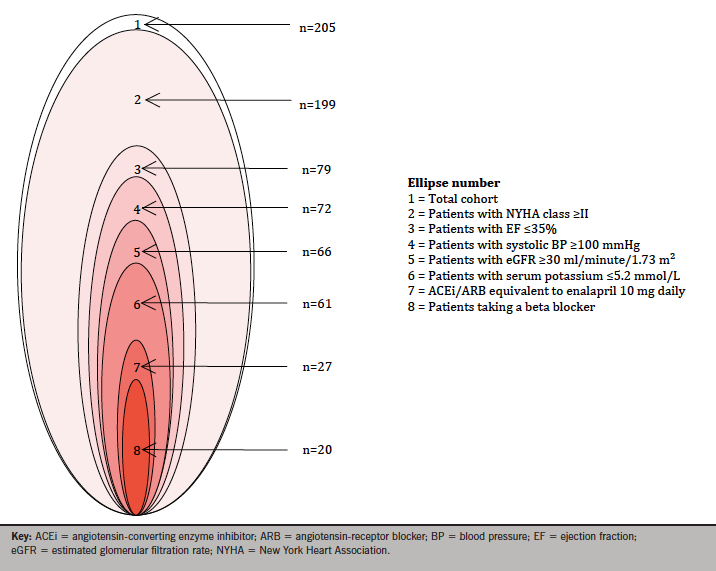
In a total of 205 assessable patients discharged with a primary diagnosis of heart failure during the calendar year 2015, inpatient mortality was 11%, with a crude readmission rate during the year of 17%. The number of patients meeting the NICE criteria was 26 (13%). In hierarchical analysis taking the major PARADIGM-HF inclusion criteria, 20 patients (10%) patients met the inclusion criteria.
In conclusion, the findings from this audit suggest that the number of patients potentially suitable for sacubitril/valsartan therapy is low. Given the PARADIGM-HF study run-in design, the optimal dose and stability of angiotensin-converting enzyme (ACE) inhibitor and angiotensin-receptor blocker medication may need to be clarified if sacubitril/valsartan is to be commenced during or shortly after hospitalisation.
Introduction
The outcome for patients with heart failure with reduced ejection fraction (HFrEF) remains poor despite optimal evidence-based therapy with angiotensin-converting enzyme inhibitors (ACEi), beta blockers (BB) and mineralocorticoid receptor antagonists (MRA). Neprilysin is an endopeptidase that degrades vasoactive peptides including natriuretic peptide. Although increased natriuretic peptide has been shown to counter maladaptive hormonal changes in heart failure, clinical studies of neprilysin inhibition have hitherto failed to improve prognosis in people with HFrEF when used alone; or in combination with ACEi.
Sacubitril/valsartan (Entresto™) is a novel combination of the neprilysin inhibitor sacubitril with the angiotensin II receptor blocker (ARB) valsartan. The PARADIGM-HF (Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure) trial was designed to compare sacubitril/valsartan 200 mg twice daily to enalapril 10 mg twice daily, in stable community-based patients with HFrEF.1 To ensure an acceptable side effect profile of the study drugs at target doses; following screening, eligible patients underwent a single-blind run-in phase comprising enalapril 10 mg twice daily, and, thereafter, sacubitril/valsartan 200 mg twice daily, after which 8,442 entered blinded randomisation. The primary end point of death from cardiovascular cause or hospitalisation due to heart failure occurred in 21.8% of the sacubitril/valsartan and 26.5% of the enalapril group. The trial was stopped after a mean follow-up of 27 months for benefit. Sacubitril/valsartan was well tolerated. Although fewer people discontinued the study due to an adverse event (10.7% vs. 12.3%), symptomatic hypotension was more common (18.0% vs. 12%) with sacubitril/valsartan.2
In March 2016, the National Institute for Health and Care Excellence (NICE) recommended the use of sacubitril/valsartan in adult patients.3 The guidance specified that such usage should be limited to people with symptomatic chronic heart failure (New York Heart Association [NYHA] class II–IV); a left ventricular ejection fraction (EF) of 35% or less; and patients “who are already taking a stable dose ACE inhibitors or ARBs”. The guidance also recommended that treatment with sacubitril/valsartan should be started by a heart failure specialist with access to a multi-disciplinary heart failure team.
Initial use of sacubitril/valsartan is, therefore, likely to be limited to areas with access to specialist services, typically hospital care either as an inpatient or shortly after discharge. The aim of this audit was to quantify the number of people discharged from hospital with a diagnosis of heart failure suitable for sacubitril/valsartan therapy based on the NICE appraisal guidance and the design of the PARADIGM-HF trial.
Methods
Clinical information was obtained retrospectively on all patients with a primary coded diagnosis of heart failure discharged from the Conquest Hospital, Hastings, UK during the calendar year 2015. For those with multiple admissions only the first in the year was recorded.
We first recorded the proportion of patients meeting the NICE recommendation; that is, patients with NYHA class II–IV symptoms; a documented EF of ≤35% (for the purposes of the audit we also included those with a visual report of more than mild impairment within this definition) and on a stable dose of ACEi or ARB (defined as meeting the PARADIGM-HF screening criteria of ACEi or ARB therapy at a dose greater than or equivalent to 10 mg once daily of enalapril) at discharge.
We then recorded those patients meeting the additional PARADIGM-HF study inclusion criteria of a stable dose of BB (defined as a prescription of BB); an estimated glomerular filtration rate (eGFR) ≥30 ml per minute per 1.73 m2; a serum potassium (K+) ≤5.2 mmol/L; a systolic blood pressure (SBP) ≥100 mmHg and where available, a plasma N-terminal pro-B-type natriuretic peptide (NT-proBNP) of ≥400 pg/ml. Data analysis was performed using Microsoft Excel.
Results
A total of 288 patients were discharged with a primary diagnosis of heart failure during the calendar year 2015. Inpatient mortality was 11%, with a crude readmission rate during the year of 17%. There were 239 first discharges, of whom 22 were deceased and 11 discharged to end-of-life care or out of area. A single patient was excluded due to having received heart transplantation, leaving 205 discharges evaluated. The mean age was 77.7 years (range 26–103), with 58% male; 82 (40%) had an EF ≤35%. The data available for each parameter and the proportion of patients meeting the PARADIGM-HF study inclusion criteria is shown in table 1.
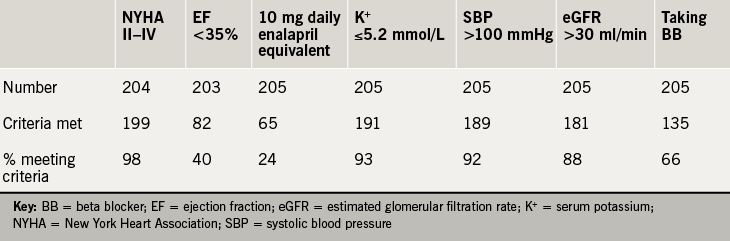
Taking the NICE guidance criteria of NYHA class II–IV, EF ≤35% and a stable dose of ACEi or ARB (recorded as a discharge dose of enalapril equivalent to or more than 10 mg daily), the number of patients meeting the criteria was 26 (13%). In hierarchical analysis, taking the major PARADIGM-HF inclusion criteria of NYHA class, EF, SBP, eGFR, serum potassium, enalapril equivalence and prescription of BB consecutively, 20 patients (10%) patients met the inclusion criteria (figure 1).
Discussion
The cohort studied comprises all heart failure discharges in first coded position and is similar to National Heart Failure Audit data (which for 2014 included 86% of all discharges in England) with average age (77.7 vs. 78 years) and male sex (58% and 56%), respectively.4 The audit population had a greater number of patients presenting with NYHA class III–IV (94% vs. 80%), and similar inpatient mortality (11% vs. 9.5%), respectively.
Applying the NICE appraisal guidance, and major inclusion criteria in the PARADIGM-HF study respectively, identified 26 (13%) and 20 (10%) patients in this population who would have been suitable for consideration for initiation of sacubitril/valsartan at or shortly after discharge. As part of their submission to the NICE technology appraisal, the manufacturer estimated that 57% of all heart failure patients in England would be suitable for sacubitril/valsartan therapy based upon population level data regarding the prevalence of HFrEF, NYHA class and eGFR. Using the same criteria in this audit, 34% of the current audit population were suitable. The difference in these proportions can be attributed primarily to the lower rate of HFrEF in this cohort, with 40% having an EF ≤35%, compared with a prevalence of more broadly defined HFrEF of 72% in the National Heart Failure Audit. In addition, although the proportion of patients with an EF ≤35% taking ACEi/ARB or BB (85% and 66%, respectively) was lower than in the National Audit (92% and 85%) this study differs from the National Audit in the tighter EF criteria, lack of exemption reporting and inclusion of every patient with a discharge diagnosis of heart failure (100% inclusion). The other dominant factor in this study was the dose of ACEi/ARB. The dose of ACEi /ARB at discharge may reflect comorbidity, commencement or down-titration during admission. In addition, the local pathway for heart failure includes post-discharge up-titration as part of an integrated service.
Limitations of the audit include the retrospective nature of the data collection, collection from a single site and the accuracy of coding, all of which may confound the analysis. Most recent serum NT-proBNP results were recorded, but were not included in the analysis, although the majority (152 of the 156 available) exceeded the PARADIGM-HF study inclusion criteria of 400 pg/ml. The definition of EF ≤35% included those with more than mild systolic impairment on visual interpretation. This afforded the advantage of permitting the inclusion of the whole cohort and moreover, insofar as this is a more ‘generous’ definition, may reflect clinical practice where an EF <40% is often taken as the threshold for evidence-based treatments. To avoid duplication, the first discharge was used for each patient during the calendar year 2015; however, this did not exclude a previous admission before 2015. In addition, this audit examined patient details at the time of discharge, whereas the PARADIGM-HF study included stable community-based patients.
Conclusion
NICE guidance supports the introduction of sacubitril/valsartan under the supervision of a heart failure specialist with access to a multi-disciplinary heart failure team. Although hospitalisation or the early post-discharge period may provide such an opportunity, the findings from this audit suggest that the number of patients potentially suitable for sacubitril/valsartan therapy is low. Given the PARADIGM-HF study run-in design, the optimal dose and stability of ACEi and ARB medication may need to be clarified if sacubitril/valsartan is to be commenced during or shortly after hospitalisation.
Conflict of interest
HFM has received advisory and consultancy fees and reimbursement for expenses and conference attendance from the pharmaceutical industry. TG and KS: none declared.
Editors’ note
See also the article by Lodge et al. in this issue.
Key messages
- The National Institute for Health and Care Excellence (NICE) recommends that sacubitril/valsartan should be started by a heart failure specialist with access to a multi-disciplinary heart failure team
- While hospitalisation or the early post-discharge period may provide an ideal opportunity to supervise the initiation of sacubitril/valsartan, only a small proportion of patients meet the NICE criteria for initiation
- A clinically useful definition of “stable dose ACE inhibitors or ARBs” is required if the hospitalisation period is to afford an opportunity to commence sacubitril/valsartan in accordance with NICE guidance
References
1. McMurray JJV, Packer M, Desai AS et al. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. http://dx.doi.org/10.1056/NEJMoa1409077
2. Novartis. Entresto Prescribing Information. Available from: https://www.pharma.us.novartis.com/product/pi/pdf/entresto.pdf [accessed 4 April 2016].
3. National Institute for Health and Care Excellence. Final appraisal document – Sacubitril valsartan for treating symptomatic chronic heart failure with reduced ejection fraction. London: NICE, April 2016. Available from: https://www.nice.org.uk/guidance/GID-TAG516/documents/html-content-2
4. British Society for Heart Failure. National Heart Failure Audit. Oxford: BSH. Available from: http://www.bsh.org.uk/resources/national-heart-failure-audit [accessed 11 April 2016].
