The first-in-class drug sacubitril/valsartan (EntrestoTM) has been recommended for use in the UK by the National Institute for Health and Care Excellence (NICE) following evidence from the PARADIGM-HF (Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure) trial. To qualify, patients should have severe left ventricular dysfunction (ejection fraction ≤35%), New York Heart Association (NYHA) grade II–IV symptomatic heart failure, and be on a stable dose of angiotensin-converting enzyme inhibitor (ACEi) or angiotensin-receptor blocker (ARB). We evaluated all patients seen in our nurse-led heart failure clinics over a six-month period to assess suitability for sacubitril/valsartan and the resulting cost implication.
Of 553 patients seen, more than two thirds (69%) were unsuitable, of whom most had an ejection fraction greater than 35%. Other reasons included hypotension, NYHA class I, renal dysfunction, intolerance of ACEi/ARB and compliance concerns. There were 49 patients who died within nine months of the study period end. Of these, most (84%) were unsuitable for sacubitril/valsartan. Compared with current local use of ACEi and ARB, switching the 174 suitable patients to sacubitril/valsartan would cost £171,816 per year.
In our real-world experience, 31–37% of patients attending a specialist nurse-led heart failure clinic may be suitable for sacubitril/valsartan therapy. While the clinical benefits of this treatment are well proven and a recent NICE technology assessment has demonstrated cost-effectiveness, the medication has significant upfront cost implications for healthcare commissioners.
Introduction
The recent PARADIGM-HF (Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure) trial provided compelling data regarding the efficacy of the first-in-class drug LCZ696, or sacubitril/valsartan (EntrestoTM, Novartis), an angiotensin-receptor neprilysin inhibitor (ARNI) that demonstrated a significant reduction in mortality and hospitalisation in heart failure patients when compared with standard treatment with angiotensin-converting enzyme inhibitors (ACEi) or angiotensin-receptor blockers (ARB).1 The subsequent consultation and recent technology assessment by the National Institute for Health and Care Excellence (NICE) has recommended that this drug be made rapidly available to heart failure patients in the UK meeting the following criteria:2
- Ejection fraction ≤35%
- Symptomatic heart failure graded New York Heart Association (NYHA) functional class II–IV
- Already on a stable dose of ACEi or ARB.
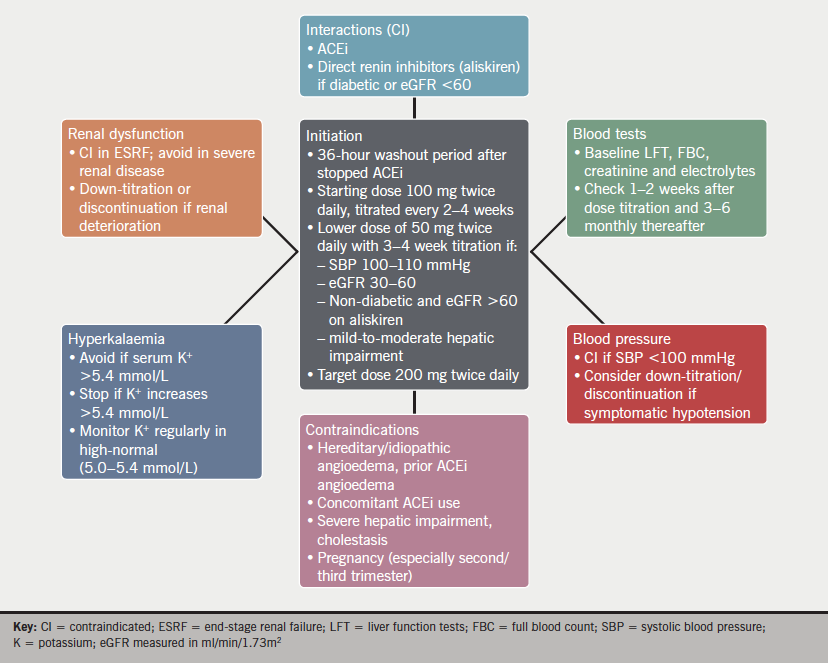
In addition, the producer of the drug, Novartis, recommends that the drug not be given to patients with a systolic blood pressure less than 100 mmHg, a prior history of angio-oedema, end-stage renal failure or severe liver dysfunction, cholestasis or biliary cirrhosis.3 Experience with patients who have estimated glomerular filtration rate (eGFR) less than 30 ml/min/1.73m2 is limited and, therefore, the drug is not recommended in this group.
In the PARADIGM-HF study, the benefits of treatment with sacubitril/valsartan over-and-above ACEi or ARB therapy were substantial and highly significant; 20% risk reduction of cardiovascular death and/or heart failure hospitalisation, 16% risk reduction of all-cause death, and 21% risk reduction of heart failure hospitalisation. Moreover, after a mandated run-in trial period to demonstrate drug tolerability, no safety concerns in relation to treatment with sacubitril/valsartan were observed in the PARADIGM-HF study.1
The cost of the drug to National Health Service (NHS) commissioning services is £91.56 per month, compared with £0.94 for 20 mg lisinopril, £2.14 for 5 mg twice-daily ramipril, £1.39 for 16 mg candesartan and £1.16 for 100 mg losartan (excluding VAT).4 The impact of the introduction of this new drug to the UK formularies has significant cost implications for primary and secondary care budgets.
We, therefore, conducted a retrospective observational study of the patients attending heart failure services within Cardiff and Vale University Health Board to evaluate the potential impact of introducing sacubitril/valsartan as proposed by NICE.
Method
This analysis was conducted at Cardiff and Vale University Health Board (University Hospital of Wales) which serves a secondary care catchment population of ~500,000. All patients attending nurse-led heart failure clinics within our unit between 1 January and 30 June 2015 were evaluated using online hospital records from clinic appointments. Data were collected regarding medication history (ACEi, ARB and mineralocorticoid-receptor antagonists [MRA]), NYHA functional class, ejection fraction, creatinine, eGFR and blood pressure (BP). From these data, an independent investigator evaluated whether they met the criteria for being commenced on sacubitril/valsartan. Reasons for unsuitability were documented and were as follows: ejection fraction >35%, unable to tolerate ACEi/ARB, eGFR <30 ml/min/1.73m2, systolic BP <100 mmHg, NYHA class I, not on stable ACEi/ARB dose, other. If multiple reasons for unsuitability were present, priority was assigned in a hierarchical fashion in the order given above. Where systolic BP was less than 110 mmHg, previous clinic blood pressures were checked and an average of three used to determine suitability. Creatinine/eGFR results and blood pressure measurements were not used where the patient was judged to be acutely decompensated, in which case the previous result, where the patient had been stable, was used. Judgements were made on a case-by-case basis where patients were poorly compliant or had comorbidities that would have been a relative contraindication to commencing sacubitril/valsartan. Data were also collected from hospital records regarding death during or following the monitoring period.
Results are presented as categorical data with number and percentage, as mean and standard deviation for parametric data and as median and inter-quartile range for non-parametric data.
Cost-analysis data was taken from published material from the PARADIGM-HF trial, from local audit and from the NHS tariff.4 Prices given exclude VAT.
Results
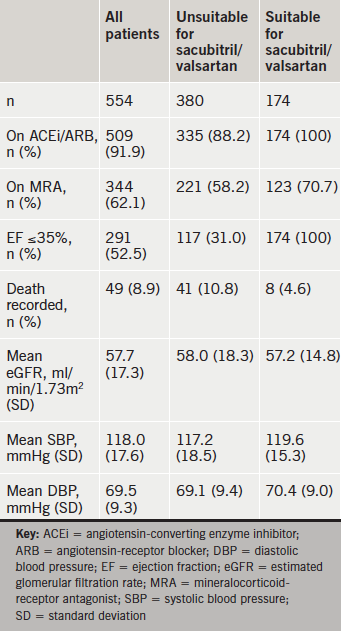
A total of 554 patients were analysed; 174 (31%) were judged to be suitable for sacubitril/valsartan (table 1). Of those who were unsuitable, the majority had an ejection fraction of greater than 35% (253 patients, 67%). The other main reasons for unsuitability were, in order of prevalence, hypotension (n=39, 10%), NYHA class I (n=21, 6%), low GFR or ongoing renal issues (n=19, 5%), intolerance of ACEi/ARB (n=13, 3%) and compliance concerns (n=3, 1%). Two patients had significant comorbidities (malignancy, deteriorating chronic confusion) and one patient had no available echocardiogram data with no evidence of left ventricular systolic dysfunction. Additionally, a further 31 patients (5.6%) were undergoing up-titration of existing heart failure medications and, therefore, may have been suitable once this was complete. If all of these patients were subsequently suitable, then 37% of patients would meet criteria set out by NICE and Novartis product recommendations.
Over 90% of those seen in the heart failure clinic were taking ACEi or ARB and nearly two thirds were on MRA. Just over half of patients had an ejection fraction of less than or equal to 35% and the majority of patients were in NYHA class II.
There were 49 (8.9%) patients who died during or since the study period. Proportionately, over twice as many deaths were recorded in the group that were unsuitable than the group that was suitable for sacubitril/valsartan.
Specific drugs were not recorded. However, locally, 75% patients are known to take ACEi and 25% ARBs. Ramipril is the ACEi of choice at a cost of £25.68/patient/year and candesartan the agent of choice for ARBs at £16.68/patient/year, giving a weighted mean of £23.43/patient/year. The average annual cost per patient is, therefore, £987.45 (cost of sacubitril/valsartan [£1,010.88 for 12 packs/year] minus cost of ACEi/ARB [£23.43 per year]) and the extra cost for all 174 patients would be £171,816.30 compared with standard treatment.
The PARADIGM-HF trial had the primary end point of a composite of death from cardiovascular causes or hospitalisation for heart failure. The number of patients who would need to be treated (NNT) over 27 months to prevent one primary event, one death from cardiovasular causes and hospitalisation for heart failure, are 21, 32 and 36, respectively.1
From our current eligible patients we estimate that treatment (for 27 months) with sacubitril/valsartan would prevent five patient deaths from cardiovascular causes and prevent 4–5 patient admissions for heart failure.
The NICE appraisal committee concluded that the most probable incremental cost-effectiveness ratio (ICER) for sacubitril/valsartan compared with ACEi was about £26,000 per quality-adjusted life year (QALY) gained.2 Using NICE costing for heart failure admissions (£2,013–£3,228) the reduction in heart failure hospital admissions for our existing patients, potentially, would save approximately £10,000–£16,000 over 27 months.
Discussion
Less than one-third of the patients in heart failure clinics in Cardiff and Vale University Health Board were suitable for sacubitril/valsartan as assessed between January and June 2015. A further 6% of patients may have been suitable at a later date once a stable dose of their ACEi or ARB had been achieved.
A significant proportion of patients in heart failure clinics are not able to tolerate standard optimal therapy due to blood pressure or renal problems. This is at odds with the population studied in the PARADIGM-HF trial, who were all required to tolerate an ACEi or ARB before being entered for the trial.
Heart failure continues to carry high mortality with nearly one in 11 patients from the cohort having died less than a year after the end-date of the sample. The discovery of a clinical treatment that reduces mortality is, therefore, a welcome addition to the current arsenal of heart failure treatments, since no drug treatment has claimed this honour for over 20 years. However, it is worth noting that the highest proportion of deaths was in those patients unsuitable for sacubitril/valsartan, suggesting that this population is more frail and that drug developments, such as this, may be of little benefit to the highest-risk patients.
Over one-half of patients studied had an ejection fraction of greater than 35%. This includes patients in whom prior severe systolic dysfunction was due to ameliorable causes (coronary disease, alcohol ingestion) as well as those who had received a biventricular pacemaker. However, it is now well-recognised that a significant proportion of patients with heart failure have normal ejection fraction, which is reflected in our sample. At present, these patients are not suitable for sacubitril/valsartan according to NICE recommendations.2 The PARAMOUNT (Prospective comparison of ARNI with ARB on Management Of heart failUre with preserved ejectioN fracTion) trial, which studied this patient group, was not powered to show outcome benefit, however, trials examining morbidity and mortality outcomes are ongoing in this patient population.5
The cost implication of this medication is significant. In our health board alone, for patients seen in only a six-month period, it is equivalent to nearly £180,000 for one year of treatment. Should guidelines be extended to include patients with higher or even normal ejection fractions, the extra cost would be considerably higher still.
Acknowledgements
The authors would like to thank Sonia Oliver and Hayley Rose for help with data collection.
Conflict of interest
FML is funded by a fellowship grant from St. Jude Medical. JP, TG and ZY: none declared.
Editors’ note
See also the article by Green et al. in this issue.
Key messages
- Approximately one-third of patients from our sample were suitable for sacubitril/valsartan
- Mortality from heart failure remains significant, but many patients at highest risk are unsuitable for evidence-based therapies
- Further treatments suitable for angiotensin-converting enzyme inhibitor (ACEi) and angiotensin-receptor blocker (ARB)-intolerant patients are required for the significant number of patients for whom sacubitril/valsartan is not an option
- The cost implication of this medication is significant and requires careful review and budgeting at the health board level
References
1. McMurray JJ, Packer M, Desai AS et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. http://dx.doi.org/10.1056/NEJMoa1409077
2. National Institute for Health and Care Excellence. Appraisal consultation document. Sacubitril valsartan for treating symptomatic chronic heart failure with reduced ejection fraction. London: NICE, December 2015. Available from: https://www.nice.org.uk/guidance/TA388/documents/appraisal-consultation-document
3. Novartic Pharmaceuticals UK Ltd. Entresto film-coated tablets. Summary of Product Characteristics. Available at: https://www.medicines.org.uk/emc/medicine/31244
4. NHS Prescription Services. National Health Service Drug Tariff for England and Wales. 2016. Available at: http://www.nhsbsa.nhs.uk/PrescriptionServices/4940.aspx
5. Solomon SD, Zile M, Pieske B et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet 2012;380:1387–95. http://dx.doi.org/10.1016/S0140-6736(12)61227-6
