Wearable and smartphone-based activity and heart rate (HR) monitors are becoming increasingly common, with around 80 million devices in use in 2017. Wearable and smartphone-based devices may be dedicated HR monitors or part of an activity tracker system. One of the main aims of these devices is to encourage exercise and increase fitness, which is clearly desirable in a society with high levels of inactivity and obesity. These devices provide individuals with large amounts of data including HR information. This may, therefore, give an opportunity to document or diagnose arrhythmias. Undiagnosed atrial fibrillation is a common problem and is associated with a huge burden of potentially preventable stroke. Wearable HR monitors may provide the opportunity to identify these individuals and allow them to receive stroke prevention treatment. However, the consumer fitness market is unregulated and the manufacturers emphasise that their devices are not intended to be used for detecting heart rhythm problems. The reliability, sampling frequency and algorithms for the HR data these devices provide are hugely variable. Detected ‘abnormalities’ may inform clinical decision making but it may also trigger unnecessary anxiety and costly investigations in healthy people.
Introduction
The world’s first wireless heart rate (HR) monitor was available in 1982.1 Since then, the use of these devices has expanded hugely with estimates of 80 million health-related wearables in use in 2017.2 These devices may be wrist worn including Apple Watch, Fitbit and Garmin watches (using photoplethysmography, PPG), ear bud headphones (PPG), chest strap-based heart rate monitors (using electrocardiography) or smartphone apps, which use the device camera to measure HR. It is becoming increasingly common for patients in cardiology clinics to have access to these data. Data from chest strap HR monitors are reasonably well validated in controlled study conditions,3 but it is recognised in fitness forums4 that abnormal ‘spikes’ or ‘dropouts’ are still common in real-world use. Wearable wrist monitors vary in their accuracy, particularly on exercise,5 and the proprietary nature of their various sampling and processing algorithms creates further challenges when attempting to interpret data. In some cases, patients’ HR data may provide useful clues to the likely diagnosis. It is also increasingly common for referrals and investigations to be instigated solely due to readings from these devices, which may well be unnecessary. Specific new technology may support clinicians in managing the extra workload that may be generated in response to patient recorded HR data.
The technology
Chest strap HR monitor
The chest strap monitor consists of a monitoring unit containing sensing electrodes and a transmitter, which is attached to a strap worn around the chest (or occasionally integrated into tight fitting clothing). This records the cardiac electrical activity in a similar way to conventional electrocardiography (ECG) and transmits, usually via Bluetooth or ANT (a proprietary data transfer protocol similar to Bluetooth), to a device such as a watch, smartphone or fitness computer. In order for the device to sense appropriately, good skin contact is required and some devices require water or gel to enhance conductivity.
The chest strap monitor has been validated in various settings including at rest;3 on walking, running, dancing;6 and on cycling.7,8 These studies revealed a high level of correlation to ECG monitoring. However, in the real world, anecdotal evidence of inaccuracies, particularly ‘spikes’ and ‘dropouts’, which can occur due to poor electrode contact or environmental interference, is abundant.4 Wearable chest strap HR monitors are widely used in exercise training and are still considered the gold standard in this setting.
Wearable activity trackers
There has been a rapid increase in the availability and use of wearable activity trackers with a huge number of different commercially available devices with a range of prices, sensors and capabilities. Some of the best known devices include wrist worn devices, such as the Apple, Fitbit and Garmin watches. These devices monitor activity using accelerometers and can also record data, such as HR, temperature, skin conductivity, GPS and altitude. Similar technology is available in headphones, e.g. Jabba. Most wearables with HR monitors today use a method called photoplethysmography (PPG) to measure HR. PPG makes use of low-intensity infrared light. When the light travels through biological tissues it is absorbed by bones, skin pigments and both venous and arterial blood. Since light is more strongly absorbed by blood than the surrounding tissues, the changes in blood flow can be detected by PPG sensors as changes in the intensity of light, and this can be translated into a HR. The fit of the device and skin factors, such as temperature, tattoos, skin tone, moisture and even motion, may affect the detection.
While the accuracy of chest strap-based monitors is well validated, the relative infancy and variety of wearable technology means the reliability of their data is less clear. There have been several small studies evaluating HR monitoring using various wearable devices5,9,10 compared with chest strap monitors. Wang et al.5 evaluated four wearable devices (Apple watch, Mio Fuse, Fitbit Charge HR and Basis Peak) against chest strap HR monitoring in 50 healthy individuals exercising on a treadmill. They demonstrated variations in the HR measurements of up to 40 bpm while exercising. There was also significant variation between devices, with some tending to overestimate and some underestimating HR. The Apple watch and Mio device performed best. Stahl et al.9 carried out a similar study evaluating six different wearable devices (Scosche Rhythm, Mio Alpha, Fitbit Charge HR, Basis Peak, Microsoft Band and TomTom Runner Cardio). They demonstrated a strong correlation between the heart rates estimated by various wrist-worn monitors and the Polar RS series chest strap. Interestingly, accuracy improved with higher intensity of exercise. This is in contrast to studies of older activity monitors, which showed the reverse.11 Shcherbina et al.10 have the largest study comparing the accuracy of seven monitors with continuous telemetry in 60 healthy individuals while walking, running and cycling. This study was more diverse in both its participants (testing a relatively large group of individuals with wide ranges of skin tone and body mass index [BMI]) and its data sampling (testing cycling alongside walking/running). They found variations between devices (with the Apple watch being the most accurate) and type of exercise (cycling being typically more accurate). All devices had a median error for HR below 10% across the study activities. If a threshold of <5% median error for HR was specified, this was achieved by six devices during cycling and three while walking. There remains very little data for patients with comorbidities, at extremes of age or body habitus and performing other activities, such as swimming, and more research is necessary to assess monitor usage under these conditions.
A recently presented study (mRhythm),12 evaluated the Apple watch in conjunction with a specially developed app that uses a deep-learning algorithm for atrial fibrillation (AF) detection. The initial results of the testing phase show a 98% sensitivity and 90% specificity for the detection of AF in known AF patients, pre- and post-cardioversion. However, more data are needed to assess the potential of this application.
Smartphone apps
There is a wide range of smartphone-connected cardiac monitoring devices and health apps, which may be directed to the diagnosis and prevention of cardiovascular disease.13 The smartphone alone cannot provide continuous HR monitoring, but allows users to sample their HR using the camera function of the phone. Both methods use PPG technology as described above. In contact PPG, the user places a finger on the camera and the built-in flash provides the light source for measurements to be made. In non-contact PPG, the camera is used in the classic way by holding it in front of the face without the need for direct skin contact. There is no need for a dedicated light source as ambient light is sufficient.
The accuracy of the smartphone apps varies depending on the method used, and there is even significant variation within the two subtypes. For example, in a study by Coppetti et al.,14 which tested four iOS apps in comparison to standard pulse oximetry and ECG, the non-contact apps performed significantly worse than the contact apps, and one contact app was significantly less accurate than the other. The paper suggests that possible reasons for the differences are the quality of the cameras used (as some required the use of the lower quality front-facing camera). As camera quality in smartphones is constantly improving, this application may also become more accurate. There were very few participants with pre-existing arrhythmias or comorbidities, so more research would be necessary to assess the validity of such apps in the context of pathology.
Smartphone-based contact PPG HR monitoring with the Cardiio Rhythm app has been evaluated as a tool for AF screening in primary care.15 The app showed high sensitivity and negative predictive value compared to 12-lead ECG (although positive predictive power was low at 53.1%). This study was carried out in a large, heterogeneous group with comorbidities. This type of technology may provide opportunities as a low-cost method of AF screening.
Applying the apps
There are clinical situations where the patient’s own HR recordings can be useful to aid diagnosis. It may provide data to correlate with symptoms and suggest an arrhythmic cause.
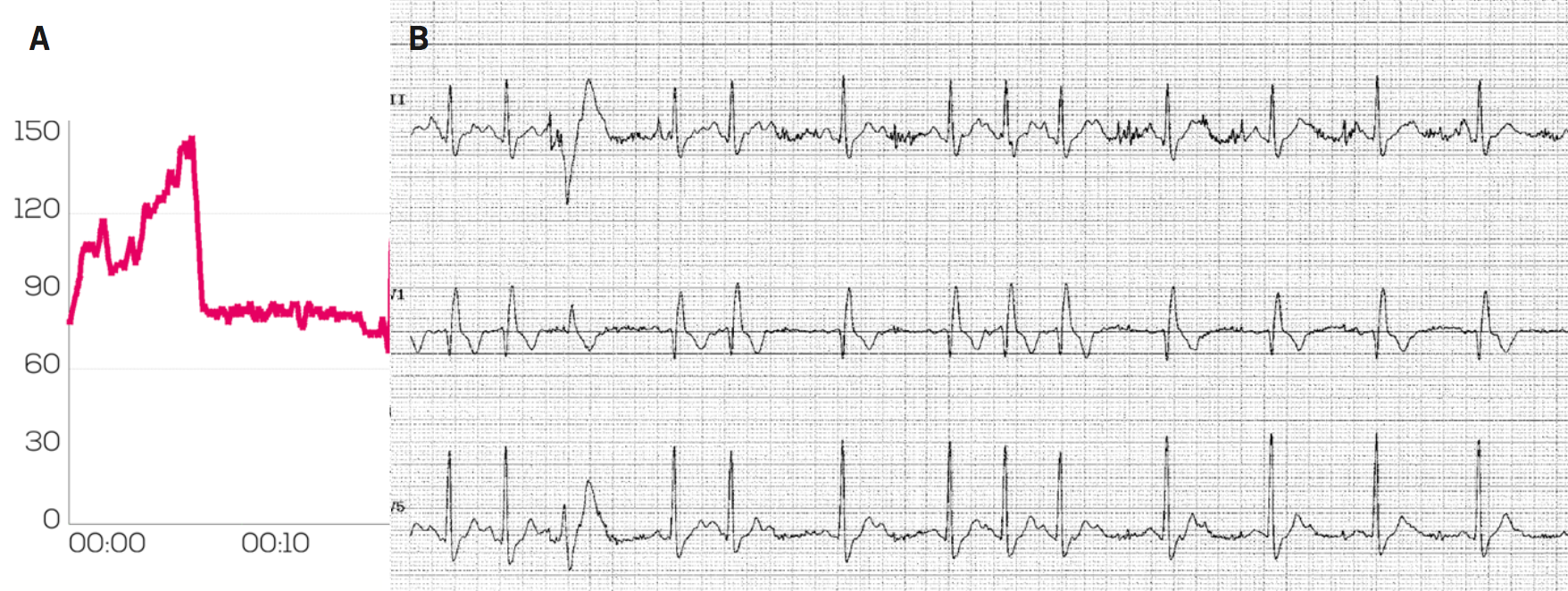
Figure 1A shows the HR monitoring data using a chest strap-based Garmin monitor from a 56-year-old man with a history of brief syncopal and presyncopal episodes occurring while cycling. The HR monitor demonstrates a sudden halving in recorded HR on exercise from approximately 150 bpm to 75 bpm around the time of his symptoms. We carried out an exercise test, which demonstrated 2:1 heart block (figure 1B) developing when he reached a sinus rate of 118 bpm, which resolved in recovery. His symptoms disappeared after implantation of a pacemaker.
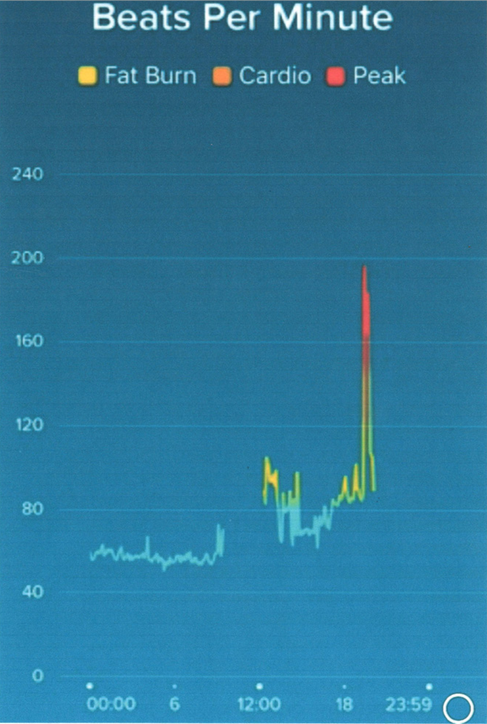
Figure 2 shows HR data from a 47-year-old woman with a 10-year history of palpitations for which she had not sought medical attention. She had recently started wearing a Fitbit and this demonstrated a sudden onset brief episode of tachycardia at a rate of approximately 195 bpm coinciding with her symptoms at a party. A local GP, also at the party, confirmed a similar HR on manual palpation. A transthoracic echo was normal, making the most likely diagnosis a supraventricular tachycardia (SVT). After being informed of this, she declined further treatment or investigation as she was not troubled by her symptoms. The data here allowed us to acknowledge that there is a likely genuine rhythm disorder and if her symptoms worsen we will be able to take appropriate steps.
Another potential application of wearable technology is in the clinical situation of AF of presumed recent onset. It is often difficult to be certain about the rhythm onset, which may have implications regarding decisions about acute cardioversion of AF lasting less than 48 hours. Supportive HR data from a wearable device can provide reassurance. Rudner et al.16 describe the use of a patient’s Fitbit data to confirm AF onset in a minimally symptomatic patient who had presented in AF following a seizure.
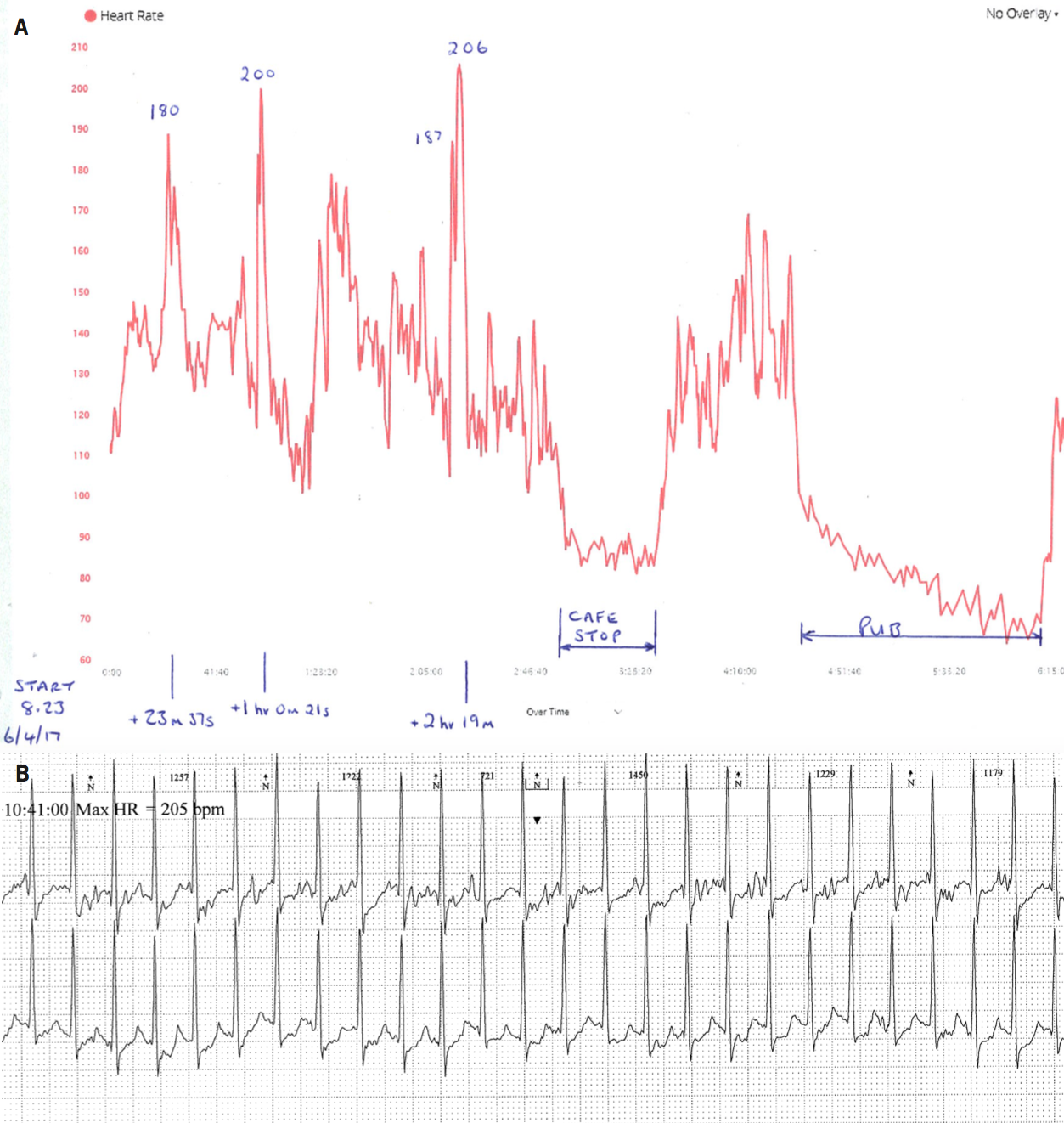
Figure 3 illustrates an example of a patient referred after consulting his GP due to concerns regarding ‘spikes’ in heart rate up to over 200 bpm recorded on his Garmin chest strap monitor while exercising (figure 3A). He was completely asymptomatic and unaware of any loss of performance at this time, but very anxious. We suspected this ‘spike’ was due to inaccuracies in the monitor, however, ambulatory ECG monitoring carried out at the same time as these traces were obtained (figure 3B) demonstrated a sudden increase in HR to 200 bpm with an alteration in P-wave morphology consistent with an atrial arrhythmia. This lasted a few seconds before a sudden change in HR and restoration of sinus tachycardia. He was asymptomatic. This man was experiencing brief episodes of atrial arrhythmia detected on exercise. The rest of his ambulatory recording and an echocardiogram were normal. We agreed that we would not commence any specific treatment due to his lack of symptoms or limitation. This is an example of the HR monitor detecting a real brief rhythm disturbance but ultimately no action was required.
Discussion
Whether clinicians like it or not, wearable monitors that provide HR and activity data will become increasingly common. Their data will be presented to clinicians who will have to integrate this into current clinical practice. However, these fitness devices are not subject to any regulatory processes and their data quality is highly variable. We hope that ‘fitness device’ manufacturers may be more open about how their devices measure and process data and adopt some industry minimum standards, but accept that this is unlikely. Data from fitness trackers may provide clues, but we need to prove any diagnosis with clinical investigations using our current well-validated medical technology. Using this approach, we may be able to identify rhythm problems earlier and give appropriate treatment, which is good for patients. However, many individuals will present without symptoms and have consulted due to changes in their HR data alone that have caused concern. It seems unavoidable that referral rates for the worried well will increase, which has a cost and time burden on primary care and cardiology services. It is often hard to resist further investigations in this group.
Perhaps developments in medical grade technology will be able to address some of the additional workload that may be created by fitness trackers. The AliveCor device (Kardia) is an external sensor that is integrated into a smartphone case (Apple or Android). When touched by the left and right hands it provides a single-lead ECG (lead 1) with near real time telemetry. It communicates wirelessly with the Kardia smartphone app and ECGs can be viewed, stored and potentially sent to healthcare professionals. It is a Food and Drug Administration (FDA) approved device, which has specific algorithms for AF detection, and the National Institute for Health and Care Excellence (NICE) have produced a briefing reviewing the device.17 It has been validated18 against 12-lead ECG for HR and intervals (e.g. QRS duration). It has also been tested for AF screening and ambulatory QT monitoring with up to 98% sensitivity and 97% specificity.19 AliveCor have also developed a Kardia Band, which is a wrist-worn device that combines wearable fitness tracker technology with smartphone rhythm analysis. It has an integrated sensor that generates a single-lead ECG when touched while being worn, and communicates wirelessly with a smartphone application, which can provide rhythm analysis. These devices, and the many others that are being developed and validated, might allow individuals to check their own ECG in response to any wearable HR monitor irregularities. They may be able to either receive electronic reassurance or capture an ECG that allows a health professional to quickly establish if there was a genuine rhythm problem and guide any further investigations.
Any increase in exercise and healthy lifestyle that the fitness tracker craze can stimulate is clearly of huge potential overall benefit to cardiovascular health. These devices may also give an opportunity to identify patients for whom further cardiac rhythm investigations and treatments may be appropriate. However, we would advocate some caution when interpreting HR data and the importance of symptom/rhythm correlation rather than responding to the numbers on the patient’s graph. Cardiology services are likely to have to adapt to allow effective and streamlined integration of technology advances into our everyday practice.
Key messages
- Fitness tracker devices can provide heart rate data, but they are unregulated and data accuracy is variable
- Patients’ own heart rate data may provide clues to a genuine rhythm problem, which needs to be confirmed using validated medical technology
- Heart rate data may lead to over investigation of the worried well, which may have significant resource implications
Conflict of interest
None declared.
References
1. Polar.com. Innovations. Available at: https://www.polar.com/uk-en/about_polar/who_we_are/innovations [accessed 1 July 2017].
2. Swan M. Sensor mania! The internet of things, wearable computing, objective metrics, and the quantified self 2.0. J Sens Actuator Netw 2012;1:217–53. https://doi.org/10.3390/jsan1030217
3. Barbosa M, da Silva N, de Azevedo F, Pastre C, Vanderlei L. Comparison of Polar® RS800G3™ heart rate monitor with Polar® S810i™ and electrocardiogram to obtain the series of RR intervals and analysis of heart rate variability at rest. Clin Physiol Funct Imaging 2016;36:112–17. https://doi.org/10.1111/cpf.12203
4. DC Rainmaker. Troubleshooting your heart rate monitor/strap HR spikes. Available at: https://www.dcrainmaker.com/2010/04/troubleshooting-your-heart-rate.html [accessed 1 July 2017].
5. Wang R, Blackburn G, Desai M et al. Accuracy of wrist-worn heart rate monitors. JAMA Cardiol 2017;2:104–06. https://doi.org/10.1001/jamacardio.2016.3340
6. Seaward BL, Sleamaker RH, McAuliffe T, Clapp JF 3rd. The precision and accuracy of a portable heart rate monitor. Biomed Instrum Technol 1990;24:37–41.
7. Kingsley M, Lewis MJ, Marson RE. Comparison of Polar 810 s and an ambulatory ECG system for RR interval measurement during progressive exercise. Int J Sports Med 2005;26:39–44. https://doi.org/10.1055/s-2004-817878
8. Engstrom E, Ottosson E, Wohlfart B, Grundstrom N, Wisen A. Comparison of heart rate measured by Polar RS400 and ECG, validity and repeatability. Adv Physiother 2012;14:115. https://doi.org/10.3109/14038196.2012.694118
9. Stahl SE, An H-S, Dinkel DM, Noble JM, Lee J-M. How accurate are the wrist-based heart rate monitors during walking and running activities? Are they accurate enough? BMJ Open Sport Exerc Med 2016;2:e000106. https://doi.org/10.1136/bmjsem-2015-000106
10. Shcherbina A, Mattsson CM, Waggott D et al. Accuracy in wrist-worn, sensor-based measurements of heart rate and energy expenditure in a diverse cohort. J Pers Med 2017;7:3. https://doi.org/10.3390/jpm7020003
11. Terbizan DJ, Dolezal BA, Albano C. Validity of seven commercially available heart rate monitors. Meas in Phys Educ Exerc Sci 2002;6:243–7. https://doi.org/10.1207/S15327841MPEE0604_3
12. Sanchez JM, Ballinger B, Olgin JE et al. AF detection and ablation outcomes: Answering questions that matter to patients. Detecting Atrial Fibrillation using a Smart Watch – the mRhythm study. Presented at: Heart Rhythm Society Annual Scientific Sessions, 10–13 May 2017. Available from: http://www.abstractsonline.com/pp8/#!/4227/presentation/11303
13. Nguyen HH, Silva JNA. Use of smartphone technology in cardiology. Trends Cardiovasc Med 2016;26:376–86. https://doi.org/10.1016/j.tcm.2015.11.002
14. Coppetti T, Brauchlin A, Muggler S et al. Accuracy of smartphone apps for heart rate measurement. Eur J Prev Cardiol 2017;24:1287–93. https://doi.org/10.1177/2047487317702044
15. Chan PH, Wong CK, Poh YC et al. Diagnostic performance of a smartphone-based photoplethysmographic application for atrial fibrillation screening in a primary care setting. J Am Heart Assoc 2016;5:e003428. https://doi.org/10.1161/JAHA.116.003428
16. Rudner J, McDougall C, Sailam V, Smith M, Sacchetti A. Interrogation of patient smartphone activity tracker to assist arrhythmia management. Ann Emerg Med 2016;68:292–4. https://doi.org/10.1016/j.annemergmed.2016.02.039
17. National Institute for Health and Care Excellence. AliveCor Heart Monitor and AliveECG app (Karelia Mobile) for detecting atrial fibrillation. London: NICE, 2015. Available from: https://www.nice.org.uk/guidance/mib35/resources/alivecor-heart-monitor-and-aliveecg-app-kardia-mobile-for-detecting-atrial-fibrillation-pdf-63499107274693 [accessed 4 July 2017].
18. Garabelli P, Stavrakis S, Po S. Smartphone-based arrhythmia monitoring. Curr Op Cardiol 2017;32:53–7. https://doi.org/10.1097/HCO.0000000000000350
19. Lau JK, Lowres N, Neubeck L et al. iPhone ECG application for community screening to detect silent atrial fibrillation: a novel technology to prevent stroke. Int J Cardiol 2013;165:193–4. https://doi.org/10.1016/j.ijcard.2013.01.220
