The paradigm for glucose control in type 2 diabetes has been based on historic and landmark studies demonstrating the unquestionable microvascular benefits of good glycaemic control.1-3 However, whether better control improves survival and prevents cardiovascular events has been less consistently shown, with one notable study, ACCORD (Action to Control Cardiovascular Risk in Type 2 Diabetes), showing an increased risk of cardiovascular death with tight control.2 Over the past few years, a number of randomised, placebo-controlled, cardiovascular outcome trials (CVOTs) testing novel glucose-lowering agents have demonstrated beneficial effects on mortality and cardiovascular events. This has prompted a change in emphasis away from solely targeting glycaemic control in diabetes, and focusing on reducing cardiovascular events and improving survival.

CVOTs
The key studies evaluated sodium-glucose co-transporter 2 (SGLT2) inhibitors – empagliflozin (EMPA-REG OUTCOME)4 and canagliflozin (CANVAS)5 – and glucagon-like peptide 1 (GLP-1) receptor agonists – liraglutide (LEADER)6 and semaglutide (SUSTAIN-6)7 – in patients with type 2 diabetes and cardiovascular disease or elevated cardiovascular risk (table 1).
In the EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients–Removing Excess Glucose) study, the use of empagliflozin resulted in 38% and 35% reductions in cardiovascular death and heart failure hospitalisation, respectively. Canagliflozin use in CANVAS (Canagliflozin Cardiovascular Assessment Study) resulted in a 33% reduction in heart failure hospitalisation, while the trial’s primary outcome, consisting of cardiovascular death, nonfatal myocardial infarction and stroke, was reduced by 14%.
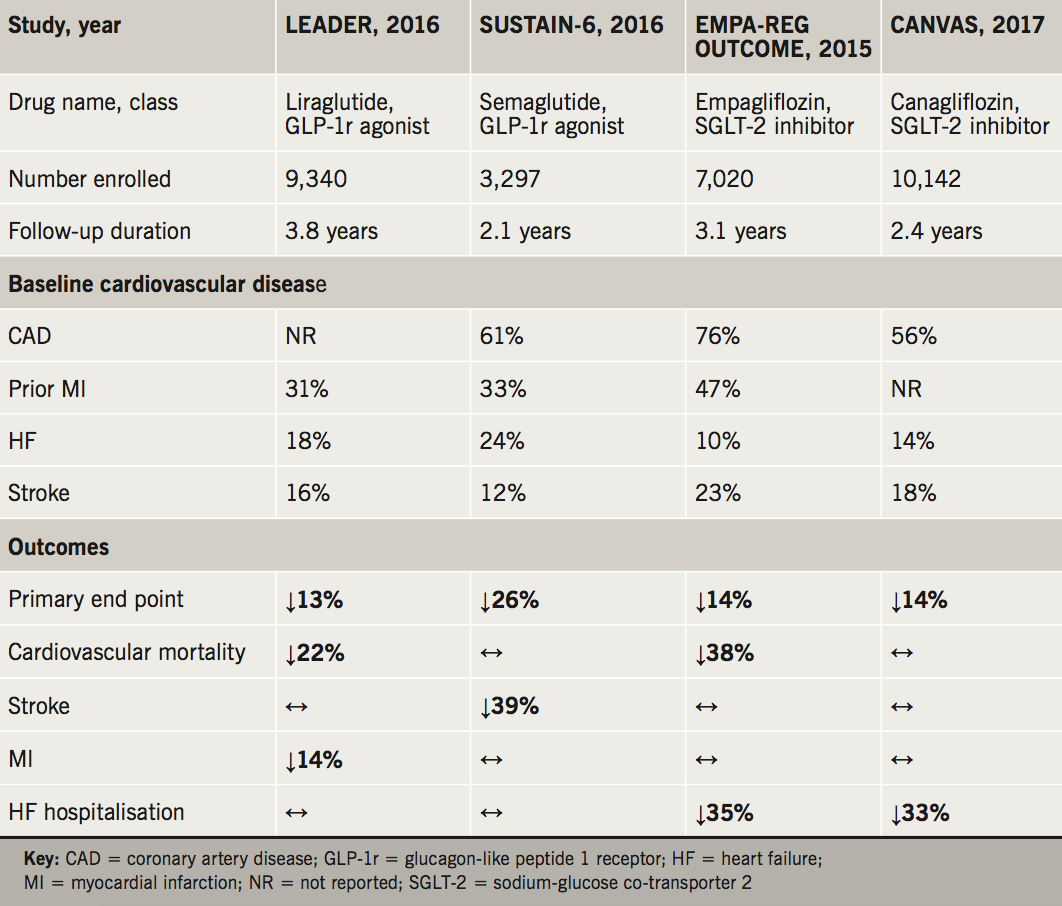
Meanwhile the GLP-1 agonists liraglutide and semaglutide have shown equally impressive improvements in major cardiovascular outcomes. Semaglutide use in SUSTAIN-6 (Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes) was associated with a 26% reduction in the primary trial outcome, with a 39% reduction in nonfatal strokes. In LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results), liraglutide resulted in a 22% reduction in cardiovascular death and 14% reduction in myocardial infarction.
Guideline updating
The beneficial effects of these drugs have been reproduced in meta-analysis,8 and taken together with results of randomised clinical trials, provide compelling evidence that using certain glucose-lowering agents can prevent early death and cardiovascular disease. The magnitudes of these benefits and the relatively short time-frames for them becoming apparent has stimulated several international organisations to update their recommendations.9-12 In November 2017, the Scottish Intercollegiate Guidelines Network (SIGN) published an updated clinical guideline on the management of type 2 diabetes incorporating results from recent CVOTs.11 One of the key changes were new recommendations on the use of SGLT2 inhibitors and GLP-1 receptor agonists with proven cardiovascular benefit in patients with type 2 diabetes and established cardiovascular disease. Furthermore, the importance of these newer drugs in reducing events in patients with cardiovascular disease will be highlighted by a joint consensus statement from the American Diabetes Association and European Association for the Study of Diabetes recommending that patients with type 2 diabetes should first be assessed for cardiovascular disease.13
Furthermore, it will go on to recommend a GLP-1 receptor agonist and a SGLT2 inhibitor as add-on therapy to metformin in patients with atherosclerotic cardiovascular disease and heart failure, respectively. This approach affirms the importance of these drugs in improving cardiovascular outcomes in these high-risk populations.
While the use of GLP-1 receptor agonists and SGLT2 inhibitors has increased within the UK since their early development, the application of recent trial evidence to clinical practice has been slow. While there may be many reasons for this, one important factor includes the clinical interpretation of current National Institute for Health and Care Excellence (NICE) guidelines for the treatment of type 2 diabetes.14 SGLT2 inhibitors are recommended in the first intensification step after metformin, but only at the end of a list of treatments that includes sulfonylureas, dipeptidyl-peptidase 4 (DPP-4) inhibitors (‘-gliptins’) and pioglitazone. GLP-1 receptor agonists, meanwhile, are only recommended if triple oral therapy is unsuccessful. The failure to highlight the potential benefits of intensification to SGLT2 inhibitors or GLP-1 receptor agonists earlier in the treatment algorithm may impact prescribing, for example in primary care and by non-cardiology or diabetes specialists.
Outstanding questions
Given that current evidence suggests that the potential survival and macrovascular benefits of good glycaemic control may take decades to accrue, the fact that these trials have demonstrated positive outcomes over a modest period of time are all the more impressive. A number of questions pertinent to the integration of these agents in guidelines remain unanswered. First, whether the beneficial cardiovascular effects are a class effect or limited only to a select number of drugs. SIGN currently recommends that only the aforementioned agents with proven cardiovascular benefit be used in patients with type 2 diabetes and cardiovascular disease. It is possible that differences in the outcomes of trials using drugs within a class may be related to study design and the populations studied, rather than true biological or pharmacological variation.
Second, it is not known where within treatment algorithms these agents should sit. Metformin is safe, effective and well-established as first-line therapy, with insufficient evidence to suggest that SGLT2 inhibitors or GLP-1 receptor agonists should replace it. The absence of direct head-to-head trial evidence comparing different drug classes makes this question harder to answer. Network meta-analysis is an approach that allows indirect comparisons to be made between treatments, and has been used to demonstrate superiority with SGLT2 inhibitors and GLP-1 receptor agonists compared with the more frequently prescribed DPP-4 inhibitors.8 Another method is to apply propensity-matching to observational studies, as was the case in the CVD-REAL study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Co-transporter-2 Inhibitors) that showed better outcomes with SGLT2 inhibitors than other oral glucose-lowering therapies.15
Third, much of our understanding of glycaemic targets (currently set at a HbA1c level of between 6.5 and 7%) is based on landmark studies, and will need to be re-evaluated in populations taking newer glucose-lowering drugs together with primary or secondary prevention therapies. Until that time, a patient-centred, individualised target should be used, minimising the harms of tight control and maximising long-term benefits.16 Furthermore, in patients who have achieved adequate glycaemic control on existing therapies, there is a paucity of data to support whether transitioning them to GLP-1 receptor agonists or SGLT2 inhibitors with de-escalation of existing treatments is appropriate.
Finally, working within the National Health Service and the constraints of limited funds, the cost-effectiveness of these new therapies will need to be carefully evaluated to ensure that benefits accrue at the population level.
Conclusion
In summary, the paradigm for the management of patients with type 2 diabetes is shifting away from focusing primarily on glycaemic control. It matters now, more than ever, how glycaemic control is reached and maintained, with several newer drugs demonstrating additional cardiovascular and survival benefits. This is particularly true for patients with atherosclerotic cardiovascular disease, where application of the available evidence into treatment algorithms has been made by most bodies, including SIGN.
Conflict of interest
None declared.
References
1. ADVANCE Collaborative Group, Patel A, MacMahon S et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–72. https://doi.org/10.1056/NEJMoa0802987
2. Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–59. https://doi.org/10.1056/NEJMoa0802743
3. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–53. https://doi.org/10.1016/S0140-6736(98)07019-6
4. Zinman B, Wanner C, Lachin JM et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–28. https://doi.org/10.1056/NEJMoa1504720
5. Neal B, Perkovic V, Mahaffey KW et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–57. https://doi.org/10.1056/NEJMoa1611925
6. Marso SP, Daniels GH, Brown-Frandsen K et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–22. https://doi.org/10.1056/NEJMoa1603827
7. Marso SP, Bain SC, Consoli A et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–44. https://doi.org/10.1056/NEJMoa1607141
8. Zheng SL, Roddick AJ, Aghar-Jaffar R et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA 2018;319:1580–91. https://doi.org/10.1001/jama.2018.3024
9. Seferovic PM, Petrie MC, Filippatos GS et al. Type 2 diabetes mellitus and heart failure: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2018;20:853–72. https://doi.org/10.1002/ejhf.1170
10. Niessner A, Tamargo J, Koller L et al. Non-insulin antidiabetic pharmacotherapy in patients with established cardiovascular disease: a position paper of the European Society of Cardiology Working Group on Cardiovascular Pharmacotherapy. Eur Heart J 2018;39:2274–81. https://doi.org/10.1093/eurheartj/ehx625
11. Scottish Intercollegiate Guidelines Network. SIGN 154. Pharmacological management of glycaemic control in people with type 2 diabetes. Edinburgh: SIGN, 2017. Available from: http://www.sign.ac.uk/assets/sign154.pdf
12. American Diabetes Association. 8. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes – 2018. Diabetes Care 2018;41(suppl 1):S73–S85. https://doi.org/10.2337/dc18-S008
13. Management of hyperglycemia in type 2 diabetes: ADA-EASD consensus report 2018. Available from: https://professional.diabetes.org/content-page/management-hyperglycemia-type-2-diabetes-ada-easd-consensus-report-2018 [accessed 2 July 2018].
14. National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. London: NICE, December 2015. Available from: https://www.nice.org.uk/guidance/ng28
15. Kosiborod M, Lam CSP, Kohsaka S et al. Cardiovascular events associated with SGLT-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL 2 study. J Am Coll Cardiol 2018;71:2628–39. https://doi.org/10.1016/j.jacc.2018.03.009
16. Abbasi J. For patients with type 2 diabetes, what’s the best target hemoglobin A1C? JAMA 2018;319:2367–9. https://doi.org/10.1001/jama.2018.5420
