Though coronary artery disease primarily occurs in those over the age of 40 years, younger individuals who use recreational drugs may be afflicted with coronary events. Cannabis is one such perilous agent that can cause myocardial infarction (MI) and is one of the most common psychoactive drugs used worldwide. Cannabis (also known as marijuana, weed, pot, dope or grass) is the most widely used illegal drug in the UK. The desired euphoric effects are immediate, as are life-threatening hazardous ones.
In this article, we briefly describe a case series of two unique but similar cases of cannabis-induced ST-elevation MI witnessed at our hospital in quick succession. We will analyse the composite pathophysiology in acute coronary syndromes provoked by cannabis and discuss the evolving legality around the use of the drug.

Background and history
In recent times, medical marijuana has been a popular topic that has necessitated legal regulation. Annual prevalence of marijuana consumption in 2017 was 147 million or roughly 2.5% worldwide,1 making it the most widely grown, distributed and consumed recreational drug.
The cannabis plant as botanical product has 480 natural components, 66 of which are classified as cannabinoids. The most commonly studied component, delta-9-tetrahydrocannabinol (THC) interacts with internal cannabinoid (CB) receptors of the human body. This activates an intricate physiological cascade, i.e. the endocannabinoid system described by Raphael Mechoulam, a regulatory system promoting balance and well-being in all mammals.2 Limited research into the benefits of cannabis has given impetus to its application as a medicinal agent.
“Weed day” is celebrated unofficially on April 20th every year. A chemical foot-print, dating back to 400 AD, marijuana’s healing properties were first described in British Medicine by Dr W O’Shaughnessy in 1842. He was an army surgeon who used it for a variety of ailments including muscle spasm, convulsions of tetanus and rheumatism. At the time, this played a partial role in overriding some of the bad press given to cannabis, such as Samuel Carey’s intoxicant depiction of cannabis in the British Pharmacopeia (1833).
Marijuana, often described as royal drug, was rumoured to be used by Queen Victoria for period pains. It was advocated for dysmenorrhoea by her personal physician Sir Robert Russell.3 Categorised as Schedule IV by the Drug Abuse convention in 1912, and banned in 1928, its medicinal use in Great Britain was outlawed in 1971. It is now regulated under Schedule I. Hemp oil, which is extracted from cannabis (THC) was allowed to be prescribed in the NHS in July 2019. It is currently awaiting the green signal from the European Medicines Agency (EMA).
Pathophysiology
The risk of developing MI has been demonstrated to be increased about fivefold in the initial first hour following cannabis smoking.4 Coronary artery spasm is the main proposed phenomenon, however, the exact hypothesised mechanism for cannabis-induced MI is likely to be much more complex. This encompasses an intricate interplay between three processes, which are increased oxygen demand, reduced oxygen supply and coronary vasospasm.
An array of mechanisms with overlapping pathophysiology have been described for marijuana-associated heart disease. Coronary vascular tone is regulated by several vasodilators and paracrine chemicals including nitric oxide (NO), endothelin-1 and prostacyclin. Nitric oxide is perhaps the most integral of these and is produced by coronary endothelial cells. Susceptibility to coronary artery spasm arises when vasodilation is impaired by endothelial damage, particularly in response to a vasoconstrictor stimulant. Smoking is well known to cause endothelial dysfunction and, when used concomitantly with cannabis, the foundation is laid for a vasospastic event. Marijuana triggers CB1 receptors inside the endothelium leading to a ROS-MAPK (reactive oxygen species – mitogen-activated protein kinase) activation cascade. This then promotes release of several reactive oxygen species (ROS) resulting in coronary smooth muscle constriction and promoting endothelial damage.5 This is just one facet, perhaps a direct consequence of cannabis and a cog in the wheel of toxicity. The pathophysiology will be expanded upon further in the discussion below.
Cases
A brief synopsis of the two cases we treated in our region are as follows. There was brisk transfer to a tertiary centre offering percutaneous coronary intervention (PCI) for both men. This was on the basis of ST-elevation on electrocardiogram (ECG) and unremitting chest pain, i.e. an indication for primary PCI.
Case 1
A 46-year-old man with a history of chronic smoking and recreational drug abuse, presented with severe chest pain following cannabis smoking hours earlier. He had no significant past medical history and was not on any prescription medications. His description of pain sounded cardiac in origin and ECG showed ST elevation in the inferior leads (II, III, aVF) as shown in figure 1. He was transferred to the tertiary cardiac hospital for consideration of primary PCI. He underwent coronary angiogram, which showed normal appearances of the coronary arteries with no flow-limiting disease (figures 2 and 3). Echocardiogram did not show any significant abnormality either and revealed a preserved biventricular systolic function. The patient most likely had coronary spasm of the right coronary artery (RCA) as a result of smoking cannabis. He was then advised to quit smoking and counselled on abstaining from cannabis and other recreational drugs.
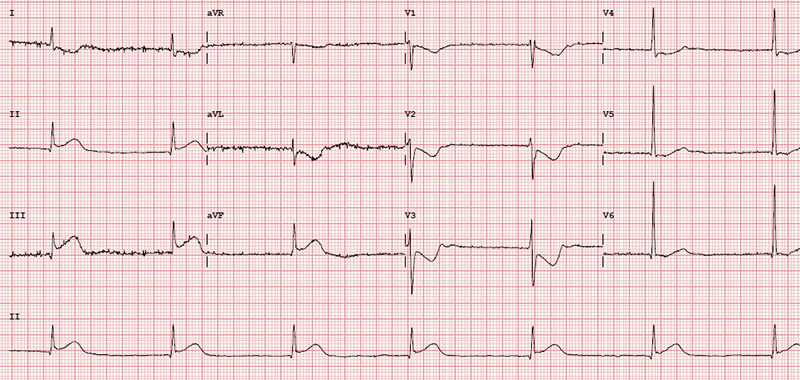
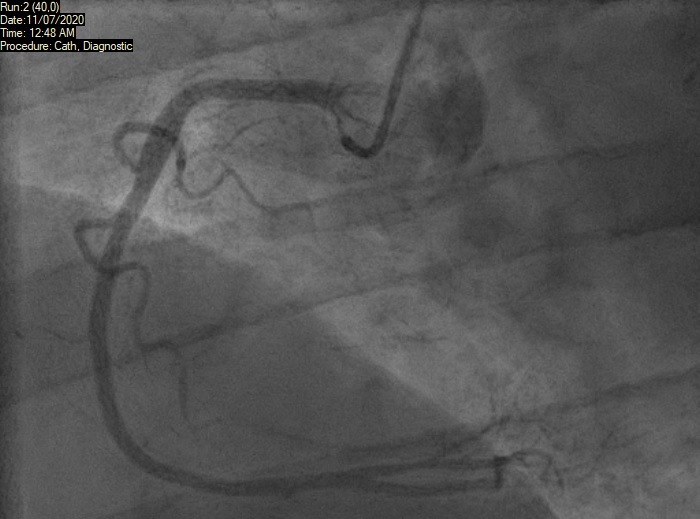
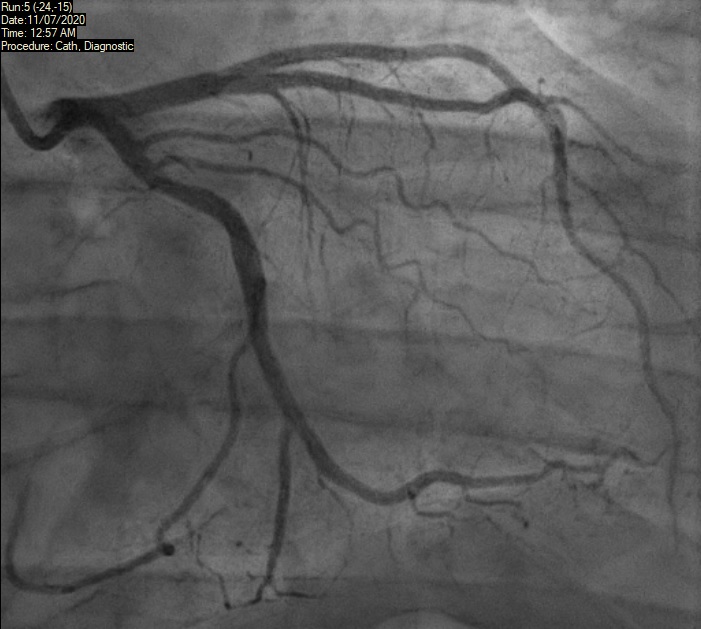
As there was no significant rise in troponin, the deduction made from his presentation and subsequent investigations was that he had not had a significant MI. Nonetheless, the initial ECG was alarming enough to warrant a diagnosis of an acute coronary syndrome related to cannabis. The presumption was that this was the result of coronary artery spasm. Given the lack of risk factors and clear trigger for the event, it was elected from him not to be put on usual secondary prevention. The focus on prevention was indeed education around the hazards of cannabis. He was, however, started on a calcium-channel blocker and planned for follow-up by his local cardiologist.
Case 2
A 39-year-old man presented to our acute district general hospital with intermittent cardiac chest pain, which started after smoking a large dose of cannabis. He provided a history of very high alcohol intake, tobacco smoking and cannabis use. He had no other significant past medical history. His ECG showed ST elevation in the anterior leads (V1–V3) as shown in figure 4. Troponin I was raised at more than 25,000 ng/L at three hours. He was subsequently transferred to a cardiac centre for an urgent coronary angiography, which showed ostial left anterior descending (LAD) artery disease (figure 5). There was a 40–50% proximal lesion with thrombus, as well as minor atheromatous changes, in the mid-segment of the vessel. There was associated significant spasm, which responded to intra-arterial nitrates. The other coronary arteries were unremarkable without any flow-limiting disease.
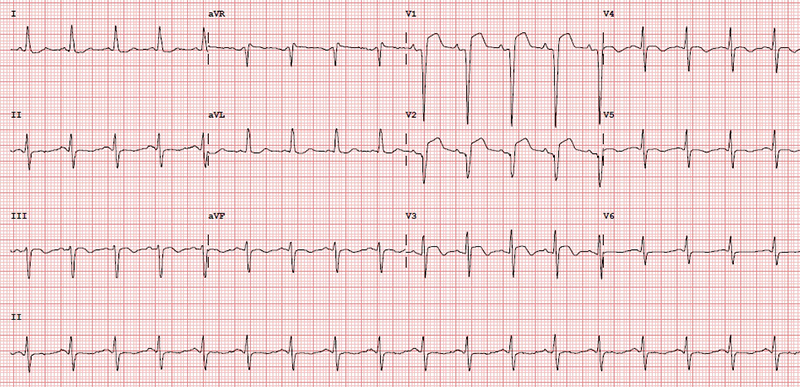
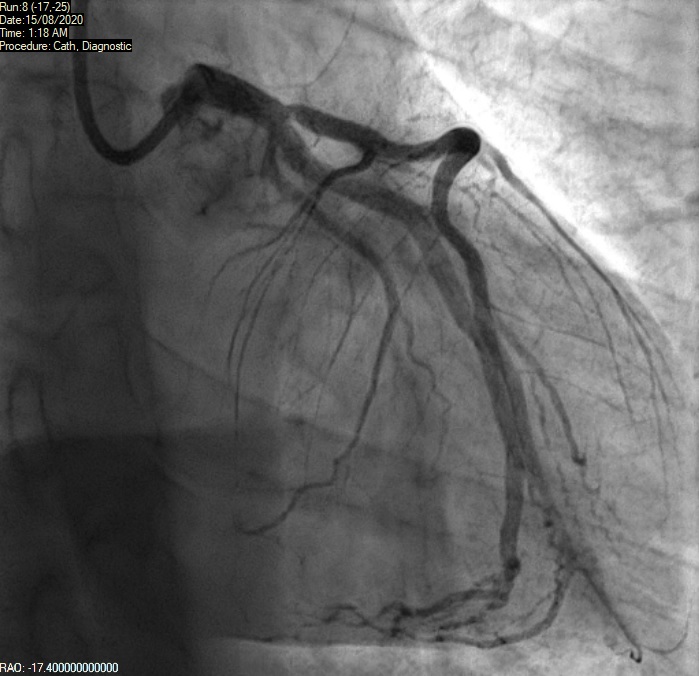
There was TIMI (Thrombolysis in Myocardial Infarction) III flow in all the vessels. Although his ostium was slightly hazy, the lesion was non-occlusive and the patient soon became pain free. Hence, he was advised to continue on medical therapy without the need for angioplasty. Left ventricular (LV) systolic function was significantly reduced on LV angiogram, later confirmed on transthoracic echocardiography. It was deemed that this man had had a plaque rupture event causing acute coronary syndrome. He was also advised to quit cannabis smoking and to reduce alcohol consumption. He was commenced on medications with dual antiplatelet therapy, angiotensin-converting enzyme (ACE) inhibitor, beta blocker, high-dose statin and a diuretic. It was arranged for him to have follow-up with cardiology and the heart failure service with a repeat echocardiogram in six weeks’ time. Cardiac magnetic resonance imaging (MRI) to further assess the aetiology of left ventricular impairment was also arranged and this confirmed ischaemic cardiomyopathy. It is quite likely that this man already had mild underlying coronary artery disease with endothelial dysfunction. He suffered a significant MI as demonstrated by grossly elevated cardiac enzymes and impaired ventricular systolic function. No doubt, this was provoked by cannabis use.
Discussion
Chest pain in association with illicit drug use is a scenario encountered by many clinicians over the course of their careers. Often the patients in question are young healthy men with no cardiovascular history. Traditionally, case reports of the clinical presentation described have depicted men with a mean age of 30 years. In fact the overwhelming majority, i.e. 92%, were male according to one literature review.5 Acute coronary syndrome and drug-induced myopericarditis are two cardiac differentials to consider. The former, as witnessed in our cases, is more serious and has the potential to be fatal. There is growing evidence to support the detrimental effects of cannabis and other illegal drugs on coronary vasculature. Some of this is perhaps anecdotal with the postulated mechanism being cannabinoid-induced coronary vasospasm and hypoperfusion. It is believed that cannabis, specifically delta-8- and delta-9-THC cause vasoconstrictor activity.6 This is just one theory and the overall mechanism is likely to be intricate and multi-factorial.
Cannabis is also known as marijuana in many parts of the Western hemisphere. Adverse, although desired, neurological effects like euphoria and drowsiness are typically seen with marijuana. It is a psychoactive drug with the main stimulant ingredient being THC. The plant from which the drug is derived contains more than 400 different chemicals. Of these, at least 66 are cannabinoids. The sheer number of constituent chemicals makes the plant difficult to study for medicinal purposes.7
Drastic myocardial hypoxaemia secondary to marijuana leads to infarction, arrhythmias or even sudden cardiac death. There is thought to be a dose-dependent pathophysiology causing a supply and demand mismatch. The effect is on the autonomic nervous system and, when consumed acutely, sympathetic-mediated tachycardia results. Parasympathetic activation is probably seen when cannabis is taken over a longer period of time. An acute-on-chronic effect manifests with hypotension and bradycardia, and, ultimately, cardiac sequelae leading to myocardial ischaemia. Nonetheless, huge amounts of cannabis taken for the first time can trigger a cascade culminating in ischaemia or death. Sustained abuse of these drugs will pose a continued danger.
Other studies suggest that marijuana causes elevated carboxyhaemoglobin levels,8 hence, another reason for hypoxaemia. Vasospasm of coronary arteries and marijuana as a trigger for MI have been elucidated to have a direct association.9 The full effects of cannabinoids on coronary circulation are not entirely understood. It is plausible that those with underlying atherosclerosis who habitually use marijuana are at highest risk of coronary events.10 The culprit lesion in a drug-induced MI is often a site with pre-existing atherosclerotic plaque.11 Endothelial dysfunction and uncontrollable vasospasm may occur in response to haemodynamic stress. CB receptors and the ROS-MAPK activation cascade are triggered as outlined above. Furthermore, cannabis could have an effect on platelet function. Another proposed mechanism is enhanced platelet aggregation at the point of focal spasmodic stenosis. THC may be the chemical component to inhibit normal platelet function, but the exact mechanism is yet to be proven.12 Theoretically, the coagulation cascade may also be implicated, as is the case with coronary events in a traditional risk-factor ridden MI. Factor VII activity may be marginally accentuated by marijuana according to preliminary reports.
Considering anecdotal case reports of cardiac events in the young who consumed cannabis, the legality of the drug, thereby, becomes a serious issue. Described as a Class B drug, cannabis is illegal for recreational use in the UK. Only very recently, as of November 2018, has the medicinal use of cannabis been legalised. This too has been based on limited, non-rigorous clinical research given logistic and ethical concerns. The efficacy and safety is yet to be determined.13 In recent years, there has been a trend to legalise even recreational marijuana in a number of US states. There is a dearth of conclusive data to support favourable medicinal use. There is perhaps a misperception among young adults (those <30 years of age) that it is harmless. In some parts of the world, cannabis is being used more than tobacco in this age group. It should, therefore, be argued that this is a public health concern. If widespread legalisation prevails, a marijuana epidemic could ensue.
Critics would say the potential recreational risks outweigh theoretical benefits. Colorado was the first state to take this step and make recreational and medicinal cannabis legal. A study in 2016, conducted by the Colorado Health Department, suggested that there was an alarming increase in marijuana-related hospital admissions since it was legalised in the state.14
It is worth reviewing, briefly, the use of medicinal marijuana. One setting in which it may be beneficial is as an anti-emetic in chemotherapy-related nausea and vomiting. It may also have an application in treating muscle spasms and chronic pain.5,15 As for the use of cannabis in treating specific diseases, Tourette syndrome and severe epilepsy are conditions where preliminary research has shown possible unproven efficacy. It seems to have an anticonvulsant effect in managing refractory seizures.16 Again, these observations are based on scant data and definitive conclusions cannot be drawn. The jury is still out on whether any potential benefits outweigh harm and the risk of misuse.
MI presenting with ST elevation (STEMI), as described in our cases, is a grave vasospastic consequence of cannabis use. ST-segment elevation seen on an ECG signifies complete occlusion of a coronary artery. In the second case, the LAD artery was implicated. Severe spasm occurred such that there must have been a 100% focal obstruction to blood flow, albeit transiently. The result of this was a significant MI with troponinaemia and ensuing heart failure, i.e. a large area of infarction. The first case was lucky in that ischaemia and focal complete coronary occlusion did not result in a discernible infarct. The RCA was the culprit vessel here but was perhaps a non-dominant artery, whereby temporary cessation of blood flow because of severe spasm did not result in myocardial death. In any case, the precarious situation he exposed himself to was clear to see and on another day he could well have been less fortunate.
Given the detrimental effects in younger individuals without underlying cardiovascular disease, those with established cardiovascular disease are surely at greater risk. Marijuana smoking compounds pre-existing endothelial dysfunction and atherosclerosis. There is a consequential increase in cardiac work and supply/demand mismatch. The mechanisms involve vasospasm, increased catecholamine levels and carboxyhaemoglobin.17
Though other modulating pathophysiology is likely, an entirely normal coronary angiogram makes spasm all but certain. Provocation testing could have a role to play in this context, but equally could be catastrophic in an already spasm-prone artery. Much is yet to be learnt about the true cardiovascular effects of cannabis. Regardless, given that it is taken to achieve a fleeting ‘high’ and consumed and shared recreationally among impressionable youths, the risks are certainly not worth taking.
Key messages
- Cannabis has been presumed to have a number of detrimental cardiac effects, particularly causative in acute coronary syndrome
- The exact mechanisms by which toxicity is seen are multi-factorial; there is thought to be a supply/demand deficit resulting from haemodynamic instability, reduced oxygen-carrying capacity, vasospasm, alteration of the clotting cascade and heightened platelet aggregation
- Vasospasm is elucidated as a key pathophysiological occurrence, leading to coronary ischaemia
- Medicinal use has gained approval in recent years with cannabis being legalised in many parts of the world for a limited number of conditions; however, it remains unproven as a treatment
- Hazards of the drug likely supersede any potential benefits
- There are public health concerns associated with the evolving legality of marijuana, such that a misperception and unawareness of danger emerges
Conflicts of interest
None declared.
Funding
None.
Patient consent
The authors had full patient consent for the write-up of the clinical cases described.
References
1. Bridgeman MB, Abazia DT. Medicinal cannabis: history, pharmacology, and implications for the acute care setting. P T 2017;42:180–8. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5312634/
2. California Cannabis Industry Association. Promoting Safe and Responsible Cannabis Use. Available from: https://www.safecannabisuse.com/ [accessed 18 November 2020].
3. Parliament UK. Select Committee on Science and Technology Ninth Report. Chapter 2 History of the use of cannabis. Available from: https://publications.parliament.uk/pa/ld199798/ldselect/ldsctech/151/15103.htm [accessed 18 November 2020].
4. Gunawardena MD, Rajapakse S, Herath J, Amarasena N. Myocardial infarction following cannabis induced coronary vasospasm. BMJ Case Rep 2014;2014:bcr2014207020. https://doi.org/10.1136/bcr-2014-207020
5. Patel KH, Kariyanna PT, Jayarangaiah A, Khondakar N, Abduraimova M, McFarlane SI. Myocardial infarction secondary to marijuana-induced coronary vasospasm. Am J Med Case Rep 2020;8:76–8. https://doi.org/10.12691/ajmcr-8-3-4
6. Yurtdas M, Aydin MK. Acute myocardial infarction in a young man; fatal blow of the marijuana: a case report. Korean Circ J 2012;42:641–5. https://doi.org/10.4070/kcj.2012.42.9.641
7. Cottencin O, Karila L, Lambert M et al. Cannabis arteritis: review of the literature. J Addict Med 2010;4:191–6. https://doi.org/10.1097/ADM.0b013e3181beb022
8. Schubart CD, Sommer IE, Fusar-Poli P, de Witte L, Kahn RS, Boks MP. Cannabidiol as a potential treatment for psychosis. Eur Neuropsychopharmacol 2014;24:51–64. https://doi.org/10.1016/j.euroneuro.2013.11.002
9. Aronow WS, Cassidy J. Effect of marihuana and placebo-marihuana smoking on angina pectoris. N Engl J Med 1974;291:65–7. https://doi.org/10.1056/NEJM197407112910203
10. Mittleman MA, Lewis RA, Maclure M, Sherwood JB, Muller JE. Triggering myocardial infarction by marijuana. Circulation 2001;103:2805–09. https://doi.org/10.1161/01.CIR.103.23.2805
11. Slavich M, Patel RS. Coronary artery spasm: current knowledge and residual uncertainties. Int J Cardiol Heart Vasc 2016;10:47–53. https://doi.org/10.1016/j.ijcha.2016.01.003
12. Jouanjus E, Lapeyre-Mestre M, Micallef J. Cannabis use: signal of increasing risk of serious cardiovascular disorders. J Am Heart Assoc 2014;3:e000638. https://doi.org/10.1161/JAHA.113.000638
13. Mach F, Montecucco F, Steffens S. Cannabinoid receptors in acute and chronic complications of atherosclerosis. Br J Pharmacol 2008;153:290–8. https://doi.org/10.1038/sj.bjp.0707517
14. Release the strains. Nat Med 2015;21:963. https://doi.org/10.1038/nm.3946
15. Whiting PF, Wolff RF, Deshpande S et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA 2015;313:2456–73. https://doi.org/10.1001/jama.2015.6358
16. Jensen B, Chen J, Furnish T, Wallace M. Medical marijuana and chronic pain: a review of basic science and clinical evidence. Curr Pain Headache Rep 2015;19:50. https://doi.org/10.1007/s11916-015-0524-x
17. Jones RT. Cardiovascular system effects of marijuana. J Clin Pharmacol 2002;42(S1):58S–63S. https://doi.org/10.1002/j.1552-4604.2002.tb06004.x
