Angiotensin-receptor blocking drugs have been shown to be an effective therapeutic strategy in a number of cardiovascular diseases. Many randomised controlled trials have demonstrated optimal doses of these drugs. We therefore investigated the doses of angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers in patients admitted to hospital. We found from a total of 60 consecutive patients, only 38% (n=23) were on the top recommended dose and the average daily dose was 63.1±4.5% of the recommended dose. This study confirms that a significant number of patients are receiving suboptimal doses of angiotensin-blocking drugs and this under-dosing is likely to result in a failure to achieve the maximal therapeutic benefit.
Introduction
Drugs that block the renin–angiotensin–aldosterone system, the angiotensin-converting enzyme inhibitors (ACEIs) and the angiotensin-receptor blockers (ARBs), have been shown to be effective in the management of hypertension, heart failure and several forms of renal disease including diabetic nephropathy. For this reason, the use of these agents is likely to increase steadily in the coming years. It is also clear from many of the randomised controlled trials that there is a dose-response curve for these agents, with higher doses being more effective.1-6 In the course of our clinical practice in acute general medicine, we observed that many of our patients, on admission to hospital, were receiving doses of these drugs that were well below those used in the published trials. We have, therefore, conducted a systematic audit of the doses of ACEI and ARB in patients admitted to hospital under our care.
Patients and methods
The City Hospital, Birmingham, is a major acute hospital in a busy industrial city, serving a catchment area of approximately 500,000 people. Acutely ill patients are admitted to a Medical Assessment Unit (MAU) pending transfer to the relevant specialist wards. We have conducted an analysis of the doses of ACEI or ARB in consecutive patients receiving these agents, who were admitted to the MAU as an emergency. Data were also collected on the medical conditions for which the drugs were being used, blood pressure and serum creatinine levels on first admission and the use of other anti-hypertensive or other cardiovascular drugs, including diuretics. The dose of the angiotensin-blocking drugs was noted and converted to a percentage of the usual top daily dose recommended in the British National Formulary No. 53, March 2007.7 These usual top daily doses were as follows: lisinopril 20 mg, ramipril 10 mg, enalapril 20 mg, perindopril 8 mg, losartan 100 mg, valsartan 160 mg, irbesartan 300 mg and candesartan 16 mg.
Results
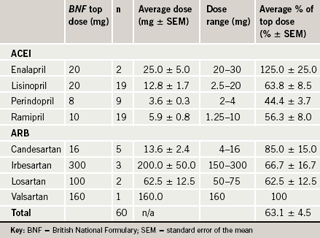
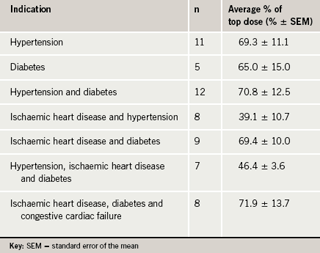
A total of 60 consecutive patients (32 males) were included in this audit. Their average age was 70.5 years (standard deviation [SD] 11.7). The indications for which an ACEI or ARB were prescribed were: hypertension 40 cases, coronary artery disease and/or heart failure 25 cases and diabetes mellitus 31 cases, with many patients having more than one indication. In none of the patients was hypertension the lone indication for receiving these drugs. The mean serum creatinine was 166.4 μmol/L (standard error of the mean [SEM] 25.2), with 15 (25%) having serum creatinine levels above 150 μmol/L. The average daily doses of the angiotensin-blocking drug are shown in table 1. Overall, the average daily dose was 63.1±4.5% of the recommended dose with a range of 12.5% to 150%. Only 38% (n=23) of patients were on the top recommended dose. Analysis of the data by indication for ACEI or ARB demonstrated that patients with a combination of ischaemic heart disease, diabetes and congestive cardiac failure were on an average of 71.9±13.7% of the recommended top dose, whereas patients with a combination of ischaemic heart disease and hypertension were on an average of 39.1±10.7% of the recommended top dose as shown in table 2. Indeed, seven of the total 60 patients who had known renal impairment were on an average of 64.3±9.2% of the recommended top dose, suggesting that renal impairment was not a likely reason for the suboptimal dosing.
Data on blood pressures were obtained but are not reported here because many may have been influenced by the acute medical condition precipitating their admission.
Discussion
Our study confirms that a significant number of patients who have been prescribed angiotensin-blocking agents are receiving suboptimal doses. The optimal dose was defined as the specific dose as recommended in theBritish National Formulary7 and in the published clinical trials. Of concern is the degree of under-dosing seen in 62% (n=37) of the patients, which does not appear to be explained by indication for angiotensin-receptor blockade. This is likely to result in patients not receiving the complete therapeutic benefit from these agents and reducing their cost-effectiveness.
These data need to be interpreted with caution, as there are several limitations to the study. The duration of treatment with the angiotensin-blocking drugs is an important factor as some patients may have only recently started angiotensin-converting enzyme (ACE) inhibition and not yet reached the optimal target dose. The doses may have been kept low because the prescribing clinician felt that adequate control of blood pressure or the symptomatic recovery of heart failure had been achieved. In some cases, the prescribing clinician may have been introducing the drugs slowly because of evidence of renal impairment. Furthermore, a number of patients may have been initiated on treatment, but subsequently lost to follow-up, before up-titration could be arranged. We have noted in the past a number of individual cases where ACEI or ARB has been started in hospital but no up-titration had been carried out by the general practitioner, as advised. Nonetheless, it appears that even allowing for these possibilities, a significant proportion of patients are not receiving adequate treatment within the remit of evidence-based medicine. A recent study of heart failure patients reported that, although 95% of patients were receiving angiotensin-blocking drugs, only 38% had achieved at least half the target dose.8 Our study, therefore, emphasises the importance of adequate dosing with angiotensin-blocking drugs and awareness within both primary and secondary care is mandatory to minimise suboptimal treatment.
Conflict of interest
None declared.
Key messages
- Angiotensin-receptor blocking drugs have been shown to be an effective therapeutic strategy in a number of cardiovascular diseases
- This study confirms that a significant number of patients are receiving suboptimal doses of angiotensin-blocking drugs
- This is likely to result in patients not receiving the complete therapeutic benefit from these agents and reducing their cost-effectiveness
References
- PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001;358:1033–41.
- Cohn JN, Tognoni G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001;345:1667–75.
- Dahlof B, Sever PS, Poulter NR et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet 2005;366:895–906.
- Lewis EJ, Hunsicker LG, Clarke WR et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001;345:851–60.
- McMurray JJ, Ostergren J, Swedberg K et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet 2003;362:767–71.
- Pfeffer MA, Swedberg K, Granger CB et al. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet 2003;362:759–66.
- BMJ Group and RPS Publishing. British National Formulary. Number 53, March 2007. London: BMJ Group and RPS Publishing, 2007.
- Cleland JG, Daubert JC, Erdmann E et al. Baseline characteristics of patients recruited into the CARE-HF study. Eur J Heart Fail 2005;7: 205–14.
