Chronic kidney disease (CKD) and heart failure with preserved ejection fraction (HFpEF) commonly co-exist. Sodium-glucose co-transporter 2 (SGLT2) inhibitors have recently emerged as key disease-modifying therapies for both conditions. In the second half of 2022, EMPA-KIDNEY (Empagliflozin in Patients with Chronic Kidney Disease) and DELIVER (Dapagliflozin Evaluation to Improve the LIVEs of Patients With PReserved Ejection Fraction Heart Failure) – two large placebo-controlled trials conducted in these populations – published their main results and expanded the evidence base in patients with and without diabetes. About one-half of each of the trials’ respective populations did not have diabetes at recruitment.1,2 Importantly, EMPA-KIDNEY represents patients with low levels of kidney function: mean estimated glomerular filtration rate (eGFR) of 37 ± 14 ml/min/1.73 m2. Both trials’ main reports were accompanied by meta-analyses in The Lancet, ensuring the new results could be reviewed in the context of the totality of evidence.
Vaduganathan et al. aggregated results from five heart failure trials,3 and the Nuffield Department of Population Health Renal Studies Group with the SGLT2 inhibitor Meta-Analysis Cardio-Renal Trialists’ Consortium combined standardised data from 13 large placebo-controlled SGLT2 inhibitor trials from three different patient populations. It included results from trials studying 42,568 patients with type 2 diabetes at high risk of atherosclerotic cardiovascular disease, 21,974 patients in heart failure trials, and 25,898 patients in CKD trials.4
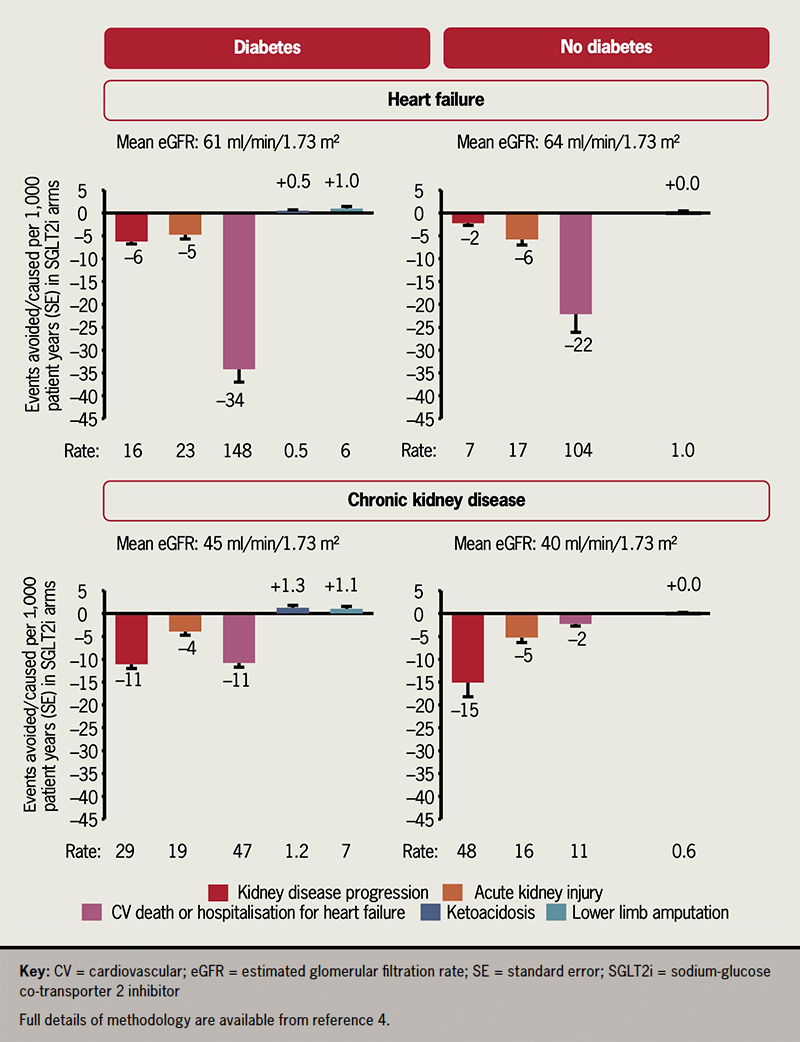
Across the 13 trials, the risk of the composite of hospitalisation for heart failure or cardiovascular death was reduced by almost one-quarter, with consistent effects in patients with and without diabetes, and across the three different trial populations.4 In the meta-analysis restricted to the heart failure trials, there were broadly consistent proportional benefits across the full range of left ventricular ejection fractions studied, and across the wide range of other subgroups.3 SGLT2 inhibitors are the first clearly effective disease-modifying therapy for HFpEF, following large trials of renin–angiotensin system (RAS) inhibitors, angiotensin-receptor/neprilysin inhibitors and mineralocorticoid receptor antagonists (MRA), which have not met their primary efficacy end points. Furthermore, evidence supporting important reductions in the risk of heart failure events when using SGLT2 inhibitors to treat acute decompensated heart failure has recently emerged from a 530-participant trial.5
The main focus of the 6,609-participant EMPA-KIDNEY trial was to establish the effect of SGLT2 inhibition on kidney disease progression. The pre-specified composite primary outcome was cardiovascular death or kidney disease progression (itself defined as initiation of maintenance dialysis or receipt of a kidney transplant, a sustained decline in eGFR to less than 10 ml/min/1.73 m2 or by at least 40% from the randomisation value, or death from kidney failure).2 Empagliflozin reduced the risk of this composite primary outcome by 28%, including a clear 27% reduction in the risk of a composite of cardiovascular death or the need to start maintenance dialysis or receive a kidney transplant.2 Cardiovascular event rates were lower than expected, and consequently 888 of the 990 participants with a primary outcome experienced kidney disease progression.2 EMPA-KIDNEY recruited 2,282 participants with an eGFR less than 30 ml/min/1.73 m2 and demonstrated remarkably consistent relative-risk reductions for its primary outcome across the full range of eGFR down to (and below) an eGFR of 20 ml/min/1.73 m2.2 Proportional effects were also similar in patients with or without a history of prior cardiovascular disease (27% of participants reported such disease at recruitment).2 When the kidney outcomes from the 13 trials were standardised to the same definitions, kidney benefits were consistent among patients with and without diabetes, and irrespective of underlying primary kidney diagnosis.4
The kidney benefits of SGLT2 inhibitors extend beyond CKD progression. Acute kidney injury (AKI) is common in patients with heart failure and in patients with CKD, and was initially considered a potential safety concern due to the natriuretic and osmotic diuretic effects of SGLT2 inhibition. However, AKI has emerged as a treatment benefit. Across all 13 trials, risk of AKI was reduced by nearly one-quarter.4 In other meta-analyses, risk of serious hyperkalaemia has been shown to be reduced by about 16%.6 SGLT2 inhibitors may, therefore, facilitate adherence to RAS inhibitors and MRA, which can cause AKI and hyperkalaemia. In EMPA-KIDNEY, the first protocol-specified recheck of kidney function was two months after initiation of empagliflozin. A routine check of eGFR (or potassium) shortly after initiation (as is common when prescribing RAS inhibitors) is not considered routinely necessary for SGLT2 inhibitors.
In EMPA-KIDNEY, empagliflozin also reduced the risk of total all-cause hospitalisations by 14% (hazard ratio [HR] 0.86, 95% confidence interval [CI] 0.78 to 0.95) – an effect that was not specific to any particular reason for hospitalisation, and was apparently irrespective of prior history of cardiovascular disease or kidney disease characteristics. SGLT2 inhibitors were also well tolerated. Ketoacidosis was a rare event in the trials despite a doubling of its risk with SGLT2 inhibitors.4 There are unanswered questions on the effects of SGLT2 inhibitors on kidney and cardiovascular outcomes in patients with type 1 diabetes, in whom the absolute risk of ketoacidosis is much higher than in patients with type 2 diabetes. There has been only a single starvation ketoacidosis event reported in a patient without diabetes during ~30,000 years of trial participant follow-up.4 The hypothesis that SGLT2 inhibitors increase amputations, raised by the CANVAS Program (CANagliflozin cardioVascular Assessment Study), has not been confirmed in the other 12 large trials.4 Similarly, the totality of evidence provides reassurances for those concerned about risk of urinary tract infection (UTI). The vast majority of UTIs in patients on an SGLT2 inhibitor in the trials were not caused by the resultant glycosuria (relative risk for UTI 1.08, 95%CI 1.02 to 1.15).4
Conclusion
In conclusion, the results from two new trials and two new meta-analyses conclusively demonstrate the cardio-protective and kidney-protective effects of SGLT2 inhibitors across the breadth of heart failure and CKD populations studied, with absolute benefits convincingly outweighing the potential harms (figure 1).4 The two new trials cement SGLT2 inhibitors as foundational therapy for both conditions, and provide clinicians with the evidence that SGLT2 inhibitors can be prescribed at low levels of eGFR, and without routinely needing additional blood monitoring following initiation.
Conflicts of interest
CTSU at the University of Oxford has a staff policy of not accepting honoraria or consultancy fees (see https://www.ctsu.ox.ac.uk/about/ctsu_honoraria_25june14-1.pdf). The authors report funds paid to their institution for the EMPA-KIDNEY trial from Boehringer Ingelheim and for the ASCEND PLUS trial from Novo Nordisk.
Funding
Funding is provided to CTSU by the UK Medical Research Council (MRC) (MC_UU_00017/3), the British Heart Foundation and Health Data Research (UK). WGH was funded by an MRC–Kidney Research UK Professor David Kerr Clinician Scientist Award (MR/R007764/1).
Data access and rights retention statement
The data presented within this manuscript are available in previously published reports. For the purpose of open access, the authors have applied a Creative Commons Attribution (CC BY) licence to any Author Accepted Manuscript version arising.
References
1. Solomon SD, McMurray JJV, Claggett B et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med 2022;387:1089–98. https://doi.org/10.1056/NEJMoa2206286
2. Herrington WG, Staplin N, Wanner C et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med 2023;388:117–27. https://doi.org/10.1056/NEJMoa2204233
3. Vaduganathan M, Docherty KF, Claggett BL et al. SGLT-2 inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomised controlled trials. Lancet 2022;400:757–67. https://doi.org/10.1016/S0140-6736(22)01429-5
4. The Nuffield Department of Population Health Renal Studies Group and the SGLT2 inhibitor Meta-Analysis Cardio-Renal Trialists’ Consortium. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet 2022;400:1788–801. https://doi.org/10.1016/S0140-6736(22)02074-8
5. Voors AA, Angermann CE, Teerlink JR et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med 2022;28:568–74. https://doi.org/10.1038/s41591-021-01659-1
6. Neuen BL, Oshima M, Agarwal R et al. Sodium-glucose cotransporter 2 inhibitors and risk of hyperkalemia in people with type 2 diabetes: a meta-analysis of individual participant data from randomized, controlled trials. Circulation 2022;145:1460–70. https://doi.org/10.1161/CIRCULATIONAHA.121.057736
