Background
Sustained reduction of elevated cholesterol (particularly low-density lipoprotein cholesterol [LDL-C]) with currently available therapies is associated with reduced atherosclerotic cardiovascular (CV) events in both primary and secondary prevention.1 Nevertheless, some individuals continue to exhibit substantial residual CV risk, which is associated with higher concentrations of atherogenic cholesterol carried by circulating triglyceride (TG)-rich lipoproteins.
The failure to reduce CV events through TG reduction in statin-treated patients with niacin, fibrates and a carboxylic acid formulation of omega-3 polyunsaturated fatty acids (n-3 PUFAs) has cast doubt on the mechanistic role of TGs in atherosclerotic cardiovascular disease (ASCVD).
This review will focus on trials of fish oils which have compared mixed formulations (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) versus EPA alone and included primary and secondary prevention populations. It will identify mechanisms of action and efficacy in CV event reduction in individuals with elevated TGs.
Clinical trials of triglyceride reduction
Three large n-3 PUFA trials showed no evidence between TG lowering and CV events – consistent with previous trials of TG-lowering agents in statin-treated patients using fibrates and niacin.1 However, there are two trials where a reduction of TG (Japan EPA Lipid Intervention Study [JELIS] 9% and the Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial [REDUCE-IT] 17%)2,3 led to reduced composite CV end points (a composite of cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, coronary revascularisation or unstable angina). A deeper exploration of the differences between trials and the active agents tested is required to inform future direction – if not TG lowering as the driver of CV event reduction, then what?
In contrast to other TG-lowering trials, the JELIS study did not prespecify a minimum TG level for study inclusion. This trial in a Japanese population showed that an ethyl ester of EPA (icosapent ethyl [IPE], 1.8 g/d) plus a statin delivered a 19% (p=0.011) reduction in a composite of CV events. The median plasma TG level at baseline of 1.7 mmol/L (153 mg/dL) probably reflected the fact that the study included patients for both primary and secondary prevention. It is worth noting that half of the patients had TG levels within the normal range and the treatment arm recorded an overall reduction of 9% compared with baseline. When outcomes were stratified to TG levels, there was a 53% reduction (p=0.04) in CV events with IPE in subjects with higher TG levels (>1.7 mmol/L [>150 mg/dL]) and low high-density lipoprotein cholesterol (HDL-C) levels (<0.45 mmol/L [<40 mg/dL]) based on a post-hoc analysis. Importantly, however, there was no relationship between the extent of TG lowering and CV events.
A higher dose of IPE (4 g/day) in REDUCE-IT led to a significant reduction of composite CV events in at-risk patients with elevated TG levels (median 2.44 mmol/L [216 mg/dL]) on maximally tolerated statins. First ischaemic events fell by 25% (p<0.001) and total (first and subsequent) ischaemic events by 31% (p<0.001). Consistent benefits were recorded across multiple pre-specified subgroups, including the primary and secondary prevention setting groups.4 The large reductions in end points exceeded that which would be expected from the 17% decrease in TG levels and again, raises questions about the mechanistic role of TGs in atherothrombotic events.5
Differences between trials
The range of CV event reductions reported from trials requires an understanding of the differences in study design between trials. These differences make direct comparisons challenging.5,6 Trials differed in the following:
- Formulations tested (EPA vs. EPA+DHA). There are clear differences between EPA and DHA at a cellular level leading to suggestions that there may be a ‘diluting’ effect of DHA. Both differ in their effects on membrane structure, rates of lipid oxidation, inflammatory biomarkers, and tissue distribution
- Dose of n-3 PUFAs tested. The absence of benefit seen in some trials may be because the dose used failed to provide the necessary threshold of achieved EPA level (~100 μg/mL) to produce clinical benefit. In the Outcomes Study to Assess STatin Residual Risk Reduction With EpaNova in HiGh CV Risk PatienTs With Hypertriglyceridemia (STRENGTH), the on-treatment levels of EPA (89.6 μg/mL) were lower than the baseline EPA levels in JELIS (97 μg/mL) and 38% lower than on-treatment levels in REDUCE-IT (144 μg/mL).1–3
- Type of placebo used. Studies varied between using no placebo or an oil substitute. Whereas STRENGTH used corn oil, the most controversial was the use of mineral oil in REDUCE-IT, raising questions about its adverse effect on CV events and thereby exaggerating the beneficial effect of IPE. A post-hoc analysis showed that the favourable effects of IPE were not influenced by fluctuations in LDL-C levels in the placebo arm.7 A sub-analysis of JELIS reported benefits to be directly related to EPA plasma levels.8 Furthermore, the US Food and Drug Administration concluded that only a small fraction, if any, of the difference could be attributed to the use of mineral oil based on comparative trials.9
- Study patient profile. All trials included patients at high risk of CV disease but some trials were exclusively in the setting of secondary prevention while others included both primary and secondary prevention settings.10
Cardiovascular risk reduction
Prespecified sub-analyses of REDUCE-IT11 reported all coronary revascularisations, subtypes of revascularisations and recurrent revascularisations. The results showed that IPE plus statins reduced the need for first and subsequent coronary revascularisations in patients with elevated TG levels and increased CV risk. Over the course of the trial (4.9 years), first coronary revascularisations were significantly reduced by 34%, with an absolute risk reduction (ARR) of 4.1% and a number needed to treat (NNT) of 24. It is interesting to note that the separation of the curves occurred early with a significant difference seen at 11 months. There were also clinically meaningful relative risk reductions (RRRs) of ≥32% in time-to-first occurrences of elective, urgent or emergent revascularisations as individual or composite end points.
Looking at revascularisation subtypes, a qualitatively similar effect was seen with a significant reduction in patients undergoing percutaneous coronary intervention in the treated arm (7.7% vs. 10.9%; p<0.001) with an ARR of 3.2% and an NNT of 31. For surgical revascularisation, there was also a significant reduction in the treated group (1.9% vs. 3.0%; p=0.0005), with an ARR of 1.1% and an NNT of 87.
Recorded plasma EPA levels showed no difference at baseline but after 360 days of active treatment, there was a consistent and statistically significant elevation in patients who did not require coronary revascularisation. This is an important observation as it suggests that clinical benefit in CV event reduction is dependent on attaining a – yet to be determined – adequate plasma EPA level and may be an explanation for the failure of previous low-dose fish oil trials and those with a mixed EPA/DHA preparation to show CV benefit.
The substudy of revascularisation – once again – highlights the effectiveness of IPE in reducing CV events to be greater than the modest degree of triglyceride reduction observed, suggesting additional mechanisms of risk reduction.
Patients with type 2 diabetes and chronic kidney disease
A substudy of 4,787 patients with diabetes12 reported a 24% reduction in the total number of CV events in the IPE-treated group (764 vs. 998; p=0.0003). The greatest gain was in those with established ASCVD who were found to have a RRR of 30%, ARR of 24% and NNT of 5. The safety of IPE in patients with diabetes was similar to the total REDUCE-IT population, with a 1% increased risk of atrial fibrillation (AF) and 0.7% increased risk of bleeding events. The authors concluded that IPE had a substantial positive impact in high-risk patients with diabetes with a good safety profile.
In the renal substudy,13 there were no meaningful changes in median estimated glomerular filtration rate (eGFR) in the IPE arm across study visits. IPE treatment led to consistent reductions in both the primary and key secondary composite end points across baseline eGFR categories. Patients with an eGFR <60 mL/min/1.73 m2 recorded the largest ARR and similar RRR for the primary composite (21.8% vs. 28.9%; p=0.0002) and key secondary composite end point (16.8% vs. 22.5%; p=0.001). The greatest numeric reduction in CV-related death was seen in the lower range eGFR <60 mL/min/1.73 m2 group (7.6% vs. 10.6%; p=0.02). Adverse events were numerically but not significantly higher in the eGFR <60 mL/min/1.73 m2 group; namely AF/flutter (4.2% vs. 3.0%; p=0.17) and serious bleeding (5.4% vs. 3.6%; p=0.13). It is important to note that the increased incidence of AF and bleeding across the trial was not associated with increased ischaemic stroke events over the full duration of the trial.
Pleiotropic mechanism of action of n-3 PUFA that modulates atherosclerosis development
The consistent absence of a relationship between reductions in TG levels and CV outcomes raises questions about underlying mechanisms. Several reviews consistently reveal an absence of benefit in clinical trials wherein both EPA and DHA were tested in amounts of ~1 g/day. Several laboratory studies show a pleiotropic effect of fish oils that may have contributed to the reductions seen in CV events in some trials (figure 1). In REDUCE-IT, there was reduced lipoprotein-associated phospholipase A2, interleukin-6, high-sensitivity C-reactive protein, apolipoprotein C-III, and oxidised LDL-C concentrations compared with placebo controls.14,15
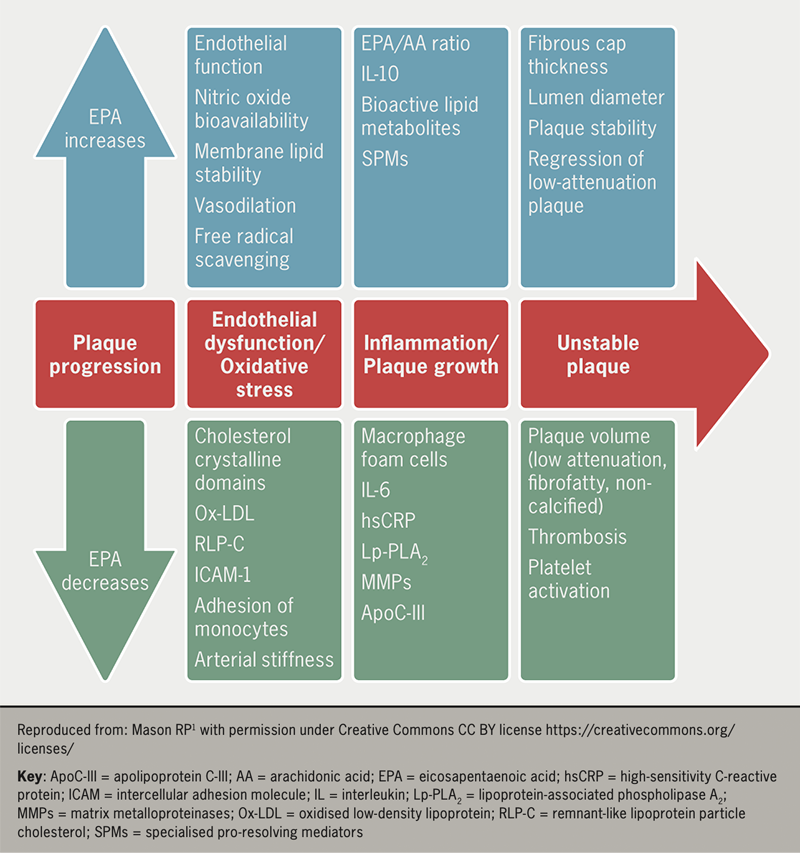
Experimental evidence of the pleiotropic effects16 of fish oil that may favourably modify atherothrombotic pathology include:
- Anti-inflammatory. Studies show n-3 PUFAs reduce inflammation and suppress expressions of inflammatory cells and inflammatory cytokines (C-reactive protein, tumour necrosis factor, interferon and interleukins)16
- Endothelial function. Trials show that fish oil consumption lowers circulating markers of endothelial dysfunction and increases flow-mediated vasodilation markers, which reflect improved endothelial function
- Antithrombotic function. n-3 PUFAs affect platelet aggregation through several mechanisms, such as the generation of thromboxane A3 and prostacyclin, that have cardioprotective and antithrombotic effects in patients treated with EPA and DHA.
Clinical evidence from trials of n-3 PUFAs lend support to an atheroprotective effect of EPA and its bioactive lipid metabolites beyond TG reduction. The effect of IPE treatment on plaque development was studied in the Effect of VASCEPA® on Improving Coronary Atherosclerosis in People With High Triglycerides Taking Statin Therapy (EVAPORATE) trial.17 Following 18 months of treatment with 4 g/day of IPE in 80 patients with high TG levels and coronary artery disease, coronary computed tomography angiography revealed a relative reduction of 17% (p<0.01) in low-attenuation plaques. The importance of this finding relates to the strong predictive ability of low-attenuation atherosclerotic plaques for fatal or non-fatal MI. This modulatory, favourable effect on plaque composition may explain the CV risk reduction seen with high-dose EPA.18 Another study using intravascular ultrasound to image plaques in coronary arteries also demonstrated a significant reduction in plaque volume in the statin and EPA combination treatment group.19
Therefore, there is mounting evidence from laboratory and clinical investigations that provide insights into the CV benefits of IPE seen in REDUCE-IT and JELIS that are independent of TG reduction.5
Adverse effects of fish oil
The most commonly reported adverse effects are minor gastrointestinal upset, such as diarrhoea, nausea, dyspepsia and abdominal discomfort, which may limit use in patients with existing digestive disorders. There is a dose-dependent increased bleeding tendency with n-3 PUFAs that is not of clinical significance, even with concurrent antiplatelet therapy.20
Several trials report an increased risk of developing AF with high-dose n-3 PUFAs. REDUCE-IT showed a near 50% increase in hospitalisations for AF in the IPE arm, whilst the STRENGTH trial was stopped early, partly because of an increased risk of AF. Two meta-analyses, one of seven randomised control trials and the second of 38 randomised control trials, reported a 25% and 26% increase in new-onset AF, respectively, but no increase in ischaemic strokes.21,22 These findings were surprising, as earlier studies had suggested that n-3 PUFAs have anti-AF effects,23 which is concerning, given that AF is associated with an increased risk of morbidities (stroke and heart failure) and mortality. However, the results of a meta-analysis of trials of fish oils should be treated with caution as they may overestimate the risk of AF because of informative censoring i.e., if n-3 PUFAs reduce mortality or delay death, or the treated patients have more time alive to acquire AF than controls, which could potentially inflate the incidence of AF in the n-3 PUFA arm.24 For this reason, the real risk of AF from n-3 PUFA supplements remains unknown and further research is warranted. Until then, as with any intervention, clinicians should weigh the risk-benefit ratio when recommending n-3 PUFA supplements for primary or secondary prevention of CV disease.
Conclusion
Numerous trials of fish oils in patients with CV disease have failed to deliver a clear message unlike, for example, trials of statins in a similar patient cohort. Therefore, it is important to examine each trial on its own merit, rather than pooling results through meta-analyses.
Additionally, it is imperative to note that firstly, the fish oil preparations that were tested varied in composition. Secondly, in earlier studies, a majority of patients were not on a statin. Thirdly, the trial populations studied differed in their inclusion of primary and/or secondary risk patients and TG levels. Lastly, where used, the choice of placebos differed between trials.
A careful dissection of each trial suggests consistency in the ability of n-3 PUFAs to reduce TG levels but no correlation between TG reduction and CV events. To realise reductions in CV events, a high dose (2–4 g/day) of purified n-3 PUFAs is necessary to maintain high plasma EPA levels. The benefits of high-dose IPE, which include reduced revascularisation and favourable plaque modification, indicate that the mechanism is likely due to its pleiotropic actions that correlate with on-treatment EPA levels. Subgroup analyses show enhanced benefit in patients with diabetes and an eGFR <60 mL/min/1.73 m2.
Whilst increased rates of AF/flutter and bleeding were seen, these did not lead to strokes or fatalities. The impressive results from REDUCE-IT prompted an update to the European Society of Cardiology guidelines for dyslipidaemia to recommend IPE for patients with CV disease and high triglyceride levels in combination with a statin.25
Key messages
- Triglycerides (TG) are associated with increased cardiovascular (CV) events; however, trials of effective TG reduction show no reduction in CV events
- High-dose purified omega-3 polyunsaturated fatty acids (n-3 PUFAs) significantly reduce CV events and revascularisation episodes independent of their effect on TG reduction
- The reduction in CV events is likely due to the pleiotropic effect of fish oils on modulating the atherosclerotic process
Conflicts of interest
AGZ has received lecture fees from Amarin. MO and HE: none declared.
Muntaser Omari
Medical Training Initiative Fellow
Holli Evans
Internal Medicine Trainee Year 2
Freeman Hospital, Newcastle upon Tyne, NE7 7DN
Azfar G Zaman
Consultant Interventional Cardiologist (Freeman Hospital, Newcastle), and Honorary Clinical Professor of Cardiology (Newcastle University)
Vascular Biology and Medicine, Newcastle University School of Medicine, Newcastle upon Tyne, NE2 4HH
([email protected])
Articles in this supplement
Introduction
Triglyceride-rich lipoproteins and their role in cardiovascular disease
REDUCE-IT: findings and implications for practice
Icosapent ethyl use in clinical practice: current and future directions
References
1. Mason RP, Eckel RH. Mechanistic insights from REDUCE-IT STRENGTHen the case against triglyceride lowering as a strategy for cardiovascular risk reduction. Am J Med 2021;134:1085–90. https://doi.org/10.1016/j.amjmed.2021.03.014
2. Yokoyama M, Origasa H, Matsuzaki M, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet 2007;369:1090–8. https://doi.org/10.1016/S0140-6736(07)60527-3
3. Bhatt DL, Steg PG, Miller M, et al.; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019;380:11–22. https://doi.org/10.1056/NEJMoa1812792
4. Bhatt DL, Steg PG, Miller M, et al.; REDUCE-IT Investigators. Effects of icosapent ethyl on total ischemic events: from REDUCE-IT. J Am Coll Cardiol 2019;73:2791–802. https://doi.org/10.1016/j.jacc.2019.02.032
5. Mason RP, Libby P, Bhatt DL. Emerging mechanisms of cardiovascular protection for the omega-3 fatty acid eicosapentaenoic acid. Arterioscler Thromb Vasc Biol 2020;40:1135–47. https://doi.org/10.1161/ATVBAHA.119.313286
6. Mason RP, Dawoud H, Jacob RF, Sherratt SCR, Malinski T. Eicosapentaenoic acid improves endothelial function and nitric oxide bioavailability in a manner that is enhanced in combination with a statin. Biomed Pharmacother 2018;103:1231–7. https://doi.org/10.1016/j.biopha.2018.04.118
7. Olshansky B, Chung MK, Budoff MJ, et al. Mineral oil: safety and use as placebo in REDUCE-IT and other clinical studies. Eur Heart J 2020;22(Suppl J):J34–48. https://doi.org/10.1093/eurheartj/suaa117
8. Pisaniello AD, Nicholls SJ, Ballantyne CM, Bhatt DL, Wong ND. Eicosapentaenoic acid: atheroprotective properties and the reduction of atherosclerotic cardiovascular disease events. EMJ 2020;5:29–36. https://www.emjreviews.com/cardiology/symposium/eicosapentaenoic-acid-atheroprotective-properties-and-the-reduction-of-atherosclerotic-cardiovascular-disease-events/
9. Food and Drug Administration. Endocrinologic and Metabolic Drugs Advisory Committee briefing document. Available at: http://epadruginitiative.com/files/FDA_Briefing_Document_for_ADCOM.pdf [accessed 31 March 2023].
10. Sharma G, Martin SS, Blumenthal RS. Effects of omega-3 fatty acids on major adverse cardiovascular events: what matters most: the drug, the dose, or the placebo? JAMA 2020;324:2262–4. https://doi.org/10.1001/jama.2020.22387
11. Peterson BE, Bhatt DL, Steg G, et al.; REDUCE-IT Investigators. Reduction in revascularization with icosapent ethyl insights from REDUCE-IT revascularization analyses. Circulation 2021;143:33–44. https://doi.org/10.1161/CIRCULATIONAHA.120.050276
12. American Diabetes Association. Substantial cardiovascular benefit from icosapent ethyl in patients with diabetes: REDUCE-IT DIABETES. 80th Scientific Session, June 12–16, 2020. Available at: https://plan.core-apps.com/tristar_ada20/abstract/2a6a17fd-a7a6-4bc9-8bf8-35847174c48d [accessed 31 March 2023].
13. Majithia A, Bhatt DL, Friedman AN, et al. Benefits of icosapent ethyl across the range of kidney function in patients with established cardiovascular disease or diabetes: REDUCE-IT RENAL. Circulation 2021;144:1750–9. https://doi.org/10.1161/CIRCULATIONAHA.121.055560
14. Bays HE, Ballantyne CM, Braeckman RA, Stirtan WG, Soni PN. Icosapent ethyl, a pure ethyl ester of eicosapentaenoic acid: effects on circulating markers of inflammation from the MARINE and ANCHOR studies. Am J Cardiovasc Drugs 2013;13:37–46. https://doi.org/10.1007/s40256-012-0002-3
15. Ballantyne CM, Bays HE, Braeckman RA, et al. Icosapent ethyl (eicosapentaenoic acid ethyl ester): effects on plasma apolipoprotein C-III levels in patients from the MARINE and ANCHOR studies. J Clin Lipidol 2016;10:635–45.e1. https://doi.org/10.1016/j.jacl.2016.02.008
16. Sherratt SCR, Libby P, Bhatt DL, Mason RP. A biological rationale for the disparate effects of omega-3 fatty acids on cardiovascular disease outcomes. Prostaglandins Leukot Essent Fatty Acids 2022;182:102450. https://doi.org/10.1016/j.plefa.2022.102450
17. Budoff MJ, Bhatt DL, Kinninger A, et al. Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides on statin therapy: final results of the EVAPORATE trial. Eur Heart J 2020;41:3925–32. https://doi.org/10.1093/eurheartj/ehaa652
18. Williams MC, Moss AJ, Dweck M, et al. Coronary artery plaque characteristics associated with adverse outcomes in the SCOT-HEART study. J Am Coll Cardiol 2019;73:291–301. https://doi.org/10.1016/j.jacc.2018.10.066
19. Watanabe T, Ando K, Daidoji H, et al.; CHERRY study investigators. A randomized controlled trial of eicosapentaenoic acid in patients with coronary heart disease on statins. J Cardiol 2017;70:537–44. https://doi.org/10.1016/j.jjcc.2017.07.007
20. Bays HE. Safety considerations with omega-3 fatty acid therapy. Am J Cardiol 2007;99:35C−43C. https://doi.org/10.1016/j.amjcard.2006.11.020
21. Gencer B, Djousse L, Al-Ramady OT, Cook NR, Manson JE, Albert CM. Effect of long-term marine ɷ-3 fatty acids supplementation on the risk of atrial fibrillation in randomized controlled trials of cardiovascular outcomes: a systematic review and meta-analysis. Circulation 2021;144:1981–90. https://doi.org/10.1161/CIRCULATIONAHA.121.055654
22. Khan SU, Lone AN, Khan MS, et al. Effect of omega-3 fatty acids on cardiovascular outcomes: a systematic review and meta-analysis. EClinicalMedicine 2021;38:100997 https://doi.org/10.1016/j.eclinm.2021.100997
23. Calò L, Martino A, Tota C. The anti-arrhythmic effects of n-3 PUFAs. Int J Cardiol 2013;170(2 suppl 1):S21–7. https://doi.org/10.1016/j.ijcard.2013.06.043
24. Samuel M, Nattel S. Fish oil supplements may increase the risk for atrial fibrillation: what does this mean? Circulation 2021;144:1991–4. https://doi.org/10.1161/CIRCULATIONAHA.121.057464
25. Mach F, Baigent C, Catapano AL, et al.; ESC Scientific Document Group. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111–88. https://doi.org/10.1093/eurheartj/ehz455

